In vitro antimicrobial efficacy of some plant extracts against multi-drug resistant Staphylococcus aureus and Streptococcus pyogenes isolated from buffalo mastitic milk
Keywords:
Bubalus bubalis, buffaloes, antimicrobial, plant extracts, multi-drug resistant, Staphylococcus aureus, Streptococcus pyogenes, buffalo mastitisAbstract
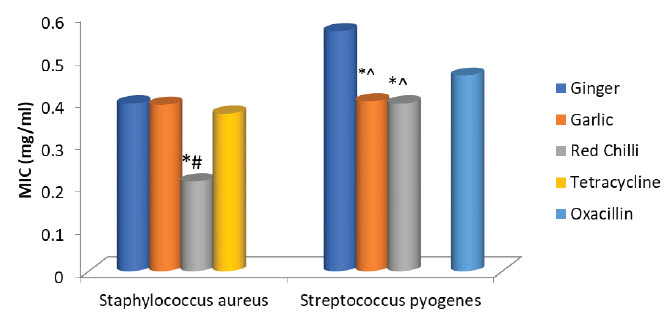
The conventional drugs used for the treatment of buffalo mastitis are losing their efficacy day by day due to increasing resistance in microbial organisms. It is therefore people nowadays are going back to use old but still quite potential remedy methods by using different herbs and shrubs for the treatment of different animal diseases including mastitis. In current investigation, three botanical extracts viz., garlic (Allium sativum L.), ginger (Zingiber officinale) and red chilies (Capsicum annuum L.) were evaluated individually and concomitantly (with ratio of 1:1) against the multidrug resistant Staphylococcus aureus and Streptococcus pyogenes isolated from buffalo mastitis. Agar well diffusion assay exhibited that red chili shown significantly (P<0.05) higher effects than garlic followed by ginger. All concentrations of red chili and 100% concentration of garlic exhibited a significantly (P<0.05) higher inhibitory effect against Strep. pyogenes comparing with other extracts and reference antibiotic oxacillin and streptomycin. Red chili as well as garlic in 75 and 100% concentrations also significantly (P<0.05) inhibited the Staph. aureus isolates comparing with ginger and reference drug. Red chili exhibited the highest inhibitory effects when combined with garlic than ginger. Garlic + red chilies showed a significantly (P<0.05) higher inhibitory effect against Staph. aureus and Strep. pyogenes as compared to other combined treatments and reference drug. Red chili as well as garlic also showed significantly (P<0.05) lower MIC (0.394 and 0.399 mg/ml respectively) against multidrug resistant Strep. pyogenes as compared to the ginger (0.564 mg/ml) and reference antibiotic oxacillin (0.460 mg/ml). Red chilies also showed significantly (P<0.05) lower (0.211 mg/ml) MIC against multidrug resistant Staph. aureus as compared to the garlic (0.391 mg/ml), ginger (0.394 mg/ml) and reference antibiotic tetracycline (0.370 mg/ml). Treatment combination based on red chili, garlic and ginger also exhibited significantly (P<0.05) lower MIC value against Staph. aureus and Strep. pyogenes as compared to ginger + garlic and reference antibiotic. This study concludes that red chili ranked 1st, garlic ranked 2nd and ginger ranked 3rd for antibacterial activity against multidrug resistant Staph. aureus and Strep. pyogenes. Treatment combination based on garlic + red chili ranked 1st, ginger + red chilies ranked 2nd and ginger + garlic ranked 3rd for antibacterial activity against multidrug resistant bacteria isolates.
Downloads
Metrics
References
Adeshina, G., S. Jibo, V.E. Agu and J.O. Ehinmidu. 2011. Antibacterial activity of fresh juices of Allium cepa and Zingiber officinale against multidrug resistant bacteria go adeshina. International Journal of Pharma and Bio Sciences, 2(2): 289-295.
Al-Anbori, D.K., M.S. Al-Nimer and A.M. Al-Weheb. 2008. Antibacterial activity of ethanolic extract of Myrtuscommunis. L leaves against salivary Mutans streptococci. Saudi Dental Journal, 20(3): 82-87.
Amber, R., M. Adnan, A. Tariq, S.N. Khan, S. Mussarat, A. Hashem, A.A. Al-Huqail, A.B.F. Al-Arjani. and E.F. Abd-Allah. 2018. Antibacterial activity of selected medicinal plants of northwest Pakistan traditionally used against mastitis in livestock. Saudi J. Biol. Sci., 25(1): 154-161. DOI: 10.1016/j.sjbs.2017.02.008
Benkeblia, N. 2004. Antimicrobial activity of essential oil extracts of various onions (Allium cepa) and garlic (Allium sativum). LWT-Food Science and Technology, 37(2): 263-268. DOI: 10.1016/j.lwt.2003.09.001
Betani, J.E., R.P. Mantovani, L.N. Barbosa, D.L.C. Stasi and A.F. Junior. 2006. Synergism between plant extract and antimicrobial drugs used on Staphylococcus aureus diseases. Mem. I. Oswaldo Cruz, 101(4): 387-390. DOI: 10.1590/s0074-02762006000400007
Bi, Y., Y.J. Wang, Y. Qin, R.G. Vallverdú, J.M. García and W. Sun. 2016. Prevalence of bovine mastitis pathogens in bulk tank milk in China. PLoS One, 11(5): e0155621. DOI: 10.1371/journal.pone.0155621
Bilal, M., G. Muhammad, F. Atif and I. Hussain. 2009. Ethno-veterinary practices of buffalo owners regarding mastitis in Faisalabad. International Journal of Applied Agricultural Sciences, 1(2): 293-296.
Bughti, A., S.H. Abro, A.A. Kamboh, R.A. Leghari, C. Kumar and S.A. Koondhar. 2017. Bacterial contamination of raw meat and butchers’ equipment in retail shops in Tando-Allahyar, Pakistan. Journal of Animal Health and Production, 5(3): 115-119. DOI: 10.17582/journal.jahp/2017/5.3.115.119
Careaga, M., E. Fernández, L. Dorantes, L. Mota, M.E. Jaramillo and H.H. Sanchez. 2003. Antibacterial activity of capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. Int. J. Food Microbiol., 83(3): 331-335. DOI: 10.1016/s0168-1605(02)00382-3
Chatterjee, S., M. Asakura, N. Chowdhury, S.B. Neogi, N. Sugimoto and S. Haldar. 2010. Capsaicin, a potential inhibitor of choleratoxin production in Vibrio cholerae. FEMS Microbiol. Lett., 306(1): 54-60. DOI: 10.1111/j.1574-6968.2010.01931.x
Deeba, F., G. Muhammad, Z. Iqbal and I. Hussain. 2009. Appraisal of ethno-veterinary practices used for different ailments in dairy animals in peri-urban areas of Faisalabad, Pakistan. Int. J. Agric. Biol., 11: 535-541. Available on: http://www.fspublishers.org/published_papers/29909_..pdf
Dego, O.K. and F. Tareke. 2003. Bovine mastitis in selected areas of southern Ethiopia. Trop. Anim. Health Pro., 35(3): 197-205. DOI: 10.1023/a:1023352811751
Durairaj, S., S. Sangeetha and P. Lakshmanaperumalsamy. 2009. In vitro antibacterial activity and stability of garlic extract at different pH and temperature. Electronic Journal of Biology, 15(1): 5-10. Available on: https://www.academia.edu/29793645/In_vitro_Antibacterial_Activity_and_Stability_of_Garlic_Extract_at_Different_pH_and_Temperature
Ebrahimi, A., K.H.P. Kheirabadi and F. Nikookhah. 2007. Antimicrobial susceptibility of environmental bovine mastitis pathogens in west central Iran. Pakistan Journal of Biological Sciences, 10(17): 3014-3016. DOI: 10.3923/pjbs.2007.3014.3016
Edwards, R. 2004. No remedy in sights for herbal ransack. New Scientist, 181: 10-11.
Freeman, D. 1997. Antimicrobial resistance: Implication for clinician., Critical Care Nursing Quarterly, 20(3): 21-35. DOI: 10.1097/00002727-199711000-00004
Getahun, K., B. Kelay, M. Bekana and F. Lobago. 2008. Bovine mastitis and antibiotic resistance patterns in selalle smallholder dairy farms, central Ethiopia. Trop. Anim. Health Pro., 40(4): 261-268. DOI: 10.1007/s11250-007-9090-5
Giday, M., Z. Asfaw and Z. Woldu. 2009. Medicinal plants of the meinit ethnic group of Ethiopia: An ethnobotanical study. J. Ethnopharmacol., 124(3): 513-521. DOI: 10.1016/j.jep.2009.05.009
Gomaa, N.F. and M.H. Hashish. 2003. The inhibitory effect of garlic (Allium sativum) on the growth of some microorganisms. Journal of the Egyptian Public Health Association, 78(5-6): 361-372.
Gull, I., M. Saeed, H. Shaukat, S.H. Aslam, Z.Q. Samra and A.M. Athar. 2012. Inhibitory effect of Allium sativum and Zingiber officinale extracts on clinically important drug resistant pathogenic bacteria. Ann. Clin. Microb. Anti., 11(8): 1-6. DOI: 10.1186/1476-0711-11-8
Habib, F., K.K. Malhi, A.A. Kamboh, R. Rind and R. Burriro. 2015. Antimicrobial susceptibility profile of Staphylococcus aureus isolates recovered from various animal species. J. Anim. Health Pro., 3(4): 99-103. DOI: 10.14737/journal.jahp/2015/3.4.99.103
Hsing, A.W., A.P. Chokkalingam, Y.T. Gao, M.P. Madigan, J. Deng, G. Gridley and J.F. Fraumeni. Allium vegetables and risk of prostate cancer: A population-based study. J. Natl. Cancer I., 94(21): 1648-1651. DOI: 10.1093/jnci/94.21.1648
Indu, M.N., A.A.M. Hatha, C. Abirosh, U. Harsha and G. Vivekanandan. 2006. Antimicrobial activity of some of the south Indian spices against serotypes of Escherichia coli, Listeria monocytogenes and Aeromonas hydrophila. Braz. J. Microbiol., 37(2): 153-158. DOI: 10.1590/S1517-83822006000200011
Iorizzi, M., V. Lanzotti, G. Ranalli, S. Marino and F. Zollo. 2002. Antimicrobial furostanol saponins from the seeds of Capsicum annuum L. var. acuminatum. J. Agr. Food Chem., 50(15): 4310-4316. DOI: 10.1021/jf0116911
Kalpoutzakis, E., I. Chinou, S. Mitaku, A.L. Skaitsounis and C. Hervala. 2000. Antibacterial, 1st ed. Calcutta: New Central Book Agency Ltd., Kolkata, India
Kalyan, K.D. 2000. An Introduction to Plant Tissue Culture, 1st ed. Calcutta: New Central Book Agency (P) Ltd., Kolkata, India
Kamboh, A.A., M.A. Arain, M.J. Mughal, A. Zaman, Z.M. Arain and A.H. Soomro. 2015. Flavonoids: Health promoting phytochemicals for animal production - A review. Journal of Animal Health and Production, 3(1): 6-13. DOI: 10.14737/journal.jahp/2015/3.1.6.13
Kamboh, A.A. and W.Y. Zhu. 2014. Individual and combined effects of genistein and hesperidin on immunity and intestinal morphometry in Lipopolysacharide-challenged Broiler chickens. Poultry Sci., 93(9): 2175-2183. DOI: 10.3382/ps.2014-03971
Kamboh, A.A. and W.Y. Zhu. 2013. Individual and combined effects of genistein and hesperidin supplementation on meat quality in meat-type Broiler chickens. J. Sci. Food. Agr., 93(13): 3362-3367. DOI: 10.1002/jsfa.6185
Lakner, L., A. Domotor, C. Toth, A. Meczker, R. Hajos, L. Kereskai, G. Szekeres, Z. Dobronte, G. Mozsik and I.L. Szabo. 2011. Capsaicin-sensitive afferentation represents an indifferent defensive pathway from eradication in patients with H. Pylori gastritis. World Journal of Gastrointestinal Pharmacology and Therapeutics, 2(5): 36-41. DOI: 10.4292/wjgpt.v2.i5.36
Lalitha, M. 2004. Manual on antimicrobial susceptibility testing. performance standards for antimicrobial testing. Twelfth Informat. Supp., 56(5): 454-456.
Malu, S.P., O. Obochi, E.N. Tawo and B.E. Nyong. 2008. Antibacterial activity and medicinal properties of ginger (Zingiber officinale). Global Journal of Pure and Applied Sciences, 15(3-4): 365-368. DOI: 10.4314/gjpas.v15i3-4.48561
Melvin, M.J., J. Jayachitra and M. Vijayapriya. 2009. Antimicrobial activity of some common spices against certain human pathogens. J. Med. Pl. Res., 3(11): 1134-1136. Available on: https://academicjournals.org/article/article1380527716_Joe%20et%20al.pdf
Mesfin, F., S. Demissew and T. Teklehaymanot. 2009. An ethnobotanical study of medicinal plants in Wonago Woreda snnpr, Ethiopia. J. Ethnobiol. Ethnomed., 5: 28.
Obasi, K.O., U.V. Emeahara, J.N. Okereke, A.C. Udebuani and M.I. Oparaigbo. 2016. The antimicrobial effects of local spices: Onions (Allium cepa, garlic (Allium sativum), ginger (Zingiber officinale), and pepper (Piper guineense) on selected pathogenic bacteria and fungus. International Journal of Science and Technology, 4: 2-84.
Omoya, F.O. and F.C. Akharaiyi. 2011. Mixture of honey and ginger extract for antibacterial assessment on some clinical isolates. International Journal of Research in Pharmaceutical and Biomedical Sciences, 2(1): 39-47.
Omoya, F.O. and F.C. Akharaiyi. 2010. A pasture honey trial for antibacterial potency on some selected pathogenic bacteria. J. Nat. Prod., 3: 5-11. Available on: http://journalofnaturalproducts.com/Volume3/2_Res_paper-1.pdf
Patmaraj, S. 2000. Drug delivery. J. Nucl. Med., 7: 91-95.
Petropoulos, S., A. Fernandes, L. Barros, A. Ciric, M. Sokovic and I.C. Ferreira. 2018. Antimicrobial and antioxidant properties of various Greek garlic genotypes. Food Chem., 245(15): 7-12. DOI: 10.1016/j.foodchem.2017.10.078
Radulovic, N., M. Denic and Z. Radic. 2010. Antimicrobial phenolic abietane diterpene from Lycopuseuropaeus L. (Lamiaceae). Bioorg. Med. Chem. Lett., 20(17): 4988-4991. DOI: 10.1016/j.bmcl.2010.07.063
Rosato, A., C. Vitali, N. De Laurentis, D. Armenise and M.A. Milillo. 2007. Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine, 14(11): 727-732. DOI: 10.1016/j.phymed.2007.01.005
Roy, J., D.M. Shakaya, P.S. Callery and J.G. Thomas. 2006. Chemical constituents and antimicrbila activity of a traditional herbal medicine containing garlic and black cumin. Afr. J. Tradit. Complem., 3: 1-7. DOI: 10.4314/ajtcam.v3i2.31151
Ruegg, P.L. 2009. Management of mastitis on organic and conventional dairy farms. J. Anim. Sci., 87(13): 43-55. DOI: 10.2527/jas.2008-1217
Sacca-Sidi, I.Y.M., G.C. Alowanou, P.A. Olounlade, V.F.G. Nadage-Dedehou and S.M. Hounzangb Adote. 2016. In vitro combined effects of Zanthoxylum zanthoxyloides and Newbouldia laevis methanolic extracts on three life-cycle stages of the parasitic nematode, Haemonchus contortus. Journal of Animal Health and Production, 4(4): 128-133. DOI: 10.14737/journal.jahp/2016/4.4.128.133
Shewit, K., H. Mekonnen, G.E. Gebremedhin and T. Samson. 2012. In vitro antimicrobial activity of some ethno-vetrinary medicinal plants traditionally used against mastitis, wound and gastrointestinal tract complication in Tigray region, Ethiopia. Asian Pacific Journal of Tropical Biomedicine, 2: 516-522.
Souza, A.B., D. Souza, M.G.M. Moreira and M.A. Moreira. 2011 Antimicrobial evaluation of diterpenes from Copaifera langsdorffii oleoresin against periodontal anaerobic bacteria. Molecul., 16(11): 9611-9619. DOI: 10.3390/molecules16119611
Stavri, M., A. Paton, B.W. Skelton and S. Gibbons. 2009. Antibacterial diterpenes from Plectranthus Ernstii. J. Nat. Prod., 72(6): 1191-1194. DOI: 10.1021/np800581s
Suleiman, M.M., L.J. Mcgaw, V. Naidoo and J.N. Eloff. 2010. Detection of antimicrobial compounds by bioautography of different extracts of leaves of selected South African tree species. Afr. J. Tradit. Complem., 7(1): 64-78. DOI: 10.4314/ajtcam.v7i1.57269
Wiart, C., T.S. Au, Y. Mohd, H. Hamimah and M. Sulaiman. 2005. 16 alpha hydroxy-(-)-kauran-19-oic: An antibacterial diterpene from sweet apple (Annona squamosa L., Annonaceae). Int. J. Pharmacol., 1(3): 296-298. DOI: 10.3923/ijp.2005.296.298




.png)








