Morphological, histopathological and secondary structure analysis of second internal transcribed spacer (ITS-2) region of Gigantocotyle explanatum (Trematoda: Paramphistomidae) in buffaloes of Pakistan
DOI:
https://doi.org/10.56825/bufbu.2022.4113161Keywords:
Bubalus bubalis, buffaloes, Gigantocotyle explanatum, histopathology, molecular characterization, PakistanAbstract
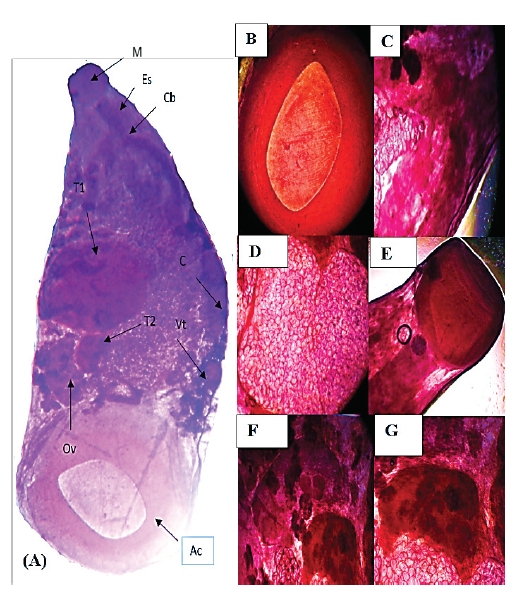
Liver infections due to amphistomes in buffaloes cause significant economic losses in livestock sector. The present study investigated the classical morphological features of adult amphistomes combined with histopathology and molecular identity in slaughtered buffaloes from Khyber Pakhtunkhwa, Pakistan. Adult amphistome were collected and morphologically characterized as Gigantocotyle explanatum. Morphometric measurement (n=50) were obtained with 12.05±1.68 mm in length and 5.77±0.81 mm in width, and the values of sagittal section was 6.35±1.03 x 2.98±0.50 mm in size. Severe bile duct pathology was observed with fibrosis of the duct wall with an irregular epithelial border, hyperplasia and inflammatory response with numerous neutrophils and eosinophils. The molecular identity of G. explanatum within Paramphistomidae was confirmed by ITS-2 rDNA sequences phylogenetic analysis based on maximum likelihood method. The genetic data based on ITS-2 secondary structure of G. explanatum consisted of four helix, Helix I, II and IV were conserved as compared with other closely related reference taxa of family Paramphistomidae and Gastrothylacidae. Helix III expressed some variations. The study concluded that rDNA ITS-2 and secondary structure information provides a guide for other researchers to determine the molecular taxonomic position of Paramphistomidae trematodes, data will support future clinical studies and control measures to reduce the amphistomiasis in buffaloes.
Downloads
Metrics
References
Barker, D.J. 1998. In utero programming of chronic disease. Clin. Sci., 95(2): 115-128.
Chaudhry, U., B. van Paridon, M. Lejeune, M.Z. Shabbir, M.I. Rashid, K. Ashraf and N. Sargison. 2017. Morphological and molecular identification of Explanatum explanatum in domestic Water buffalo in Pakistan. Veterinary Parasitology: Regional Studies and Reports, 8: 54-59. DOI: 10.1016/j.vprsr.2017.02.002
Chaudhry, U., B. van Paridon, M.Z. Shabbir, M. Shafee, K. Ashraf, T. Yaqub and J. Gilleard. 2015. Molecular evidence shows that the liver fluke Fasciola gigantica is the predominant Fasciola species in ruminants from Pakistan. J. Helminthol., 90(2): 206-213. DOI: 10.1017/S0022149X15000176
Coleman, A.W. 2007. Pan-eukaryote ITS2 homologies revealed by RNA secondary structure. Nucleic Acids Res., 35(10): 3322-3329. DOI: 10.1093/nar/gkm233
Eduardo, S.L. 1937. The taxonomy of the family Paramphistomidae with special reference to the morphology of species occurring in ruminants. Syst. Parasitol., 5: 25-79.
Gamit, A.B., P.K. Nanda, R. Bhar and S. Bandyopadhyay. 2017. Explanatum explanatum infection in the liver of Water buffalo : A slaughter house report. International Journal of Science, Environment and Technology, 6(2): 1231-1235. Available on: https://www.ijset.net/journal/1671.pdf
Gorjipoor, S., M. Moazeni and H. Sharifiyazdi. 2015. Characterization of Dicrocoelium dendriticum haplotypes from sheep and cattle in Iran based on the internal transcribed spacer 2 (ITS-2) and NADH dehydrogenase gene (nad1). J. Helminthol., 89(2): 158-164. DOI: 10.1017/S0022149X13000679
Gumasta, P., D.K. Jolhe, R.C. Ghosh, M.K. Pandey, S.K. Patel and S. Argade. 2020. Patho-morphological study of Gigantocotyle spp. infection in water buffaloes (Bubalus bubalis). J. Anim. Res., 9(4): 533-536. DOI: 10.30954/2277-940X.04.2019.6
Haque, M., C. Mohan and I. Ahmad. 2011. Natural trematode infection in liver of water buffalo (Bubalus bubalis): Histopathological investigation. Journal of Parasitic Diseases, 35(1): 50-53. DOI: 10.1007/s12639-011-0022-y
Hayashi, K., U.K. Mohanta, Y. Ohari, T. Neeraja, T.S. Singh, H. Sugiyama and T. Itagaki. 2016. Molecular characterization and phylogenetic analysis of Explanatum explanatum in India based on nucleotide sequences of ribosomal ITS2 and the mitochondrial gene nad1. J. Vet. Med. Sci., 78(11): 1745-1748. DOI: 10.1292/jvms.16-0252
Ichikawa, M., D. Kondoh, S. Bawn, N.N. Maw, L.L. Htun, M. Thein and T. Itagaki. 2013. Morphological and molecular characterization of Explanatum Explanatum from cattle and buffaloes in Myanmar. J. Vet. Med. Sci., 75(3): 309-314. DOI: 10.1292/jvms.12-0389
Iqbal, M.N., A. Muhammad, A.A. Anjum, K.A. Shahzad, M.A. Ali and S. Ali. 2014. Epidemiology of Gigantocotyle explanatum in naturally infected buffaloes. Veterinaria, 1(1): 15-18. Available on: http://thesciencepublishers.com/veterinaria/files/3-201402073-RV%20Final.pdf
Itagaki, T. and K.I. Tsutsumi. 1998. Triploid form of Fasciola in Japan: Genetic relationships between Fasciola hepatica and Fasciola gigantica determined by ITS-2 sequence of nuclear rDNA. Int. J. Parasitol., 28(5): 777-781. DOI: 10.1016/s0020-7519(98)00037-x
Jones, A. 2005. Superfamily paramphistomoidea Fischoeder, 1901. p. 221-327. In Jones, A., R.A. Bray and D.I. Gibson (eds.) Keys to The Trematoda, Wallingford, CABI Publishing, London, UK.
Joseph, N., E. Krauskopf, M.I. Vera and B. Michot. 1999. Ribosomal internal transcribed spacer 2 (ITS2) exhibits a common core of secondary structure in vertebrates and yeast. Nucleic Acids Res., 27(23): 4533-4540. DOI: 10.1093/nar/27.23.4533
Keller, A., F. Förster, T. Müller, T. Dandekar, J. Schultz and M. Wolf. 2010. Including RNA secondary structures improves accuracy and robustness in reconstruction of phylogenetic trees. Biol. Direct, 5(1): 4. DOI: 10.1186/1745-6150-5-4
Khatoon, N., M.B. Fatima and M. Samreen. 2003. Histopathological changes in the liver of buffaloes by digenetic trematode Paramphistomum cervi. Pakistan Journal of Biological Sciences, 6(17): 1540-1543. DOI: 10.3923/pjbs.2003.1540.1543
Kumar, S., G. Stecher and K. Tamura. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol., 33(7): 1870-1874. DOI: 10.1093/molbev/msw054
Laidemitt, M.R., E.T. Zawadzki, S.V. Brant, M.W. Mutuku, G.M. Mkoji and E.S. Loker. 2017. Loads of trematodes: Discovering hidden diversity of paramphistomoids in Kenyan ruminants. Parasitology, 144(2): 131-147. DOI: 10.1017/S0031182016001827
Mai, J.C. and A.W. Coleman. 1997. The internal transcribed spacer 2 exhibits a common secondary structure in green algae and flowering plants. J. Mol. Evol., 44(3): 258-271. DOI: 10.1007/pl00006143
Malik, S.I., K. Afshan and M. Qayyum. 2017. Phenotyping of amphistomes, and pathological, hematological and bile biochemical response to Gigantocotyle explanatum infection in buffaloes. Pak. J. Zool., 49(30): 979-987. DOI: 10.17582/journal.pjz/2017.49.3.979.987
Mas-Coma, S., M.A. Valero and M.D. Bargues. 2009. Chapter 2 fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control. Adv. Parasit., 69: 41-146. DOI: 10.1016/S0065-308X(09)69002-3
Mazahery, Y., J. Razmyar and N. Hoghooghi-Rad. 1994. Explanatum explanatum (Creplin, 1847) Fukui, 1929, in buffaloes in the Ahwaz area, southwest Iran. Vet. Paraitol., 55(1-2): 149-153. DOI: 10.1016/0304-4017(94)90066-3
Michot, B., N. Joseph, S. Mazan, J.P. Bachellerie, L. De Biologie, M. Eucaryote and T. Cedex. 1999. Evolutionarily conserved structural features in the ITS2 of mammalian pre-rRNAs and potential interactions with the snoRNA U8 detected by comparative analysis of new mouse sequences. Nucleic Acids Res., 27(11): 2271-2282. DOI: 10.1093/nar/27.11.2271
Mohanta, U.K., M. Ichikawa-Seki, T. Shoriki, K. Katakura and T. Itagaki. 2014. Characteristics and molecular phylogeny of Fasciola flukes from Bangladesh, determined based on spermatogenesis and nuclear and mitochondrial DNA analyses. Parasitol. Res., 113(7): 2493-2501. DOI: 10.1007/s00436-014-3898-5
Schultz, J., S. Maisel and D. Gerlach. 2005. A common core of secondary structure of the internal transcribed spacer 2 (ITS2) throughout the Eukaryota. RNA, 11(4): 361-364. DOI: 10.1261/rna.7204505
Zuker, M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res., 31(13): 3406-3415. DOI: 10.1093/nar/gkg595








.png)








