Detection of Theileria and Anaplasma spp. in carrier buffaloes (Bubalus bubalis) by polymerase chain reaction in Tamil Nadu, India
DOI:
https://doi.org/10.56825/bufbu.2022.4143426Keywords:
Bubalus bubalis, buffaloes, Theileria, Anaplasma, PCR, detection, IndiaAbstract
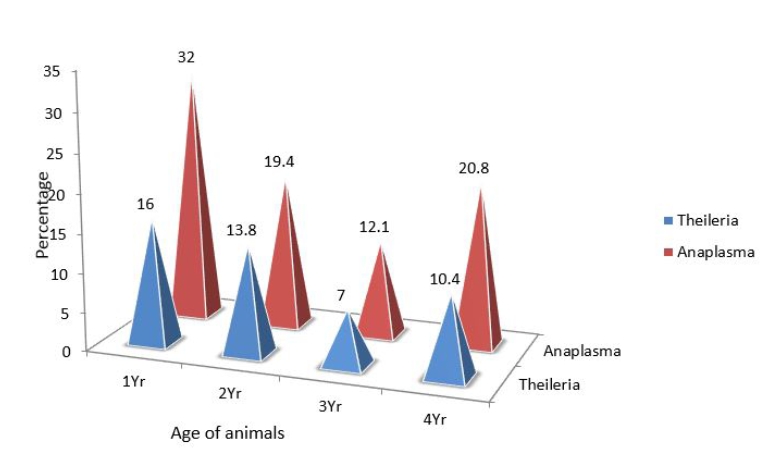
A total of 150 heparinised blood samples and equal number of blood smears were collected from buffaloes in three agro-climatic zones of Tamil Nadu viz., Cauvery delta, North - western and Southern zones to detect Theileria and Anaplasma by blood smear examination and Polymerase Chain Reaction. The blood smear examination revealed that none of animals were found to harbour any haemoprotozoan parasites. Whereas PCR detected Theileria and Anaplasma in 26.9% and 48.0%, and 10% and 15% of the animals, respectively in north- western zone and Cauvery delta, however no animal from south zone was detected positive for any protozoan. Besides, mixed infection of Theileria and Anaplasma was recorded in 8 (5.3%) animals. The analysis of data collected to determine the risk factors associated with occurrence of haemoprotozoan parasites revealed that the pure breed Murrah showed higher percentage of Theileria and Anaplasma positivity (30.7% and 46.1%) than non - descriptive breed (8.7% and 16.0%). The percentage of positivity of haemoprotozoan parasites was found to be higher among animals of 1 year, followed by 2 years and > 4 years of age.
Downloads
Metrics
References
Elhariri, M.D., R.A. Elhelw, D.A. Hamza and D.E. Soliman. 2017. Molecular detection of Anaplasma marginale in the Egyptian water buffaloes (Bubalus bubalis) based on Major surface protein 1α. Journal of the Egyptian Society of Parasitology, 47(2): 247-252. DOI: 10.21608/JESP.2017.77758
Eriks, I.S., D. Stiller and G. Palmer. 1993. Impact of persistent Anaplasma marginale
rickettsemia on tick infection and transmission. J. Clin. Microbiol., 31(8): 2091-2096. DOI: 10.1128/jcm.31.8.2091-2096.1993
Khan, M.Q., A. Zahoor, M. Jahangir and M.A. Mirza. 2004. Prevalence of blood parasites in cattle and buffaloes. Pak. Vet. J., 24(4): 193-195. Available on: http://www.pvj.com.pk/pdf-files/24_4/193-195.pdf
Kolte, S.W., S.D. Larcombe, G.S. Jadhao, S.P. Magar, G. Wathi, N.V. Kurkure, E.J. Glass and R.B. Shiels. 2017. PCR diagnosis of tick-borne pathogens in Maharashtra state, India indicates fitness cost associated with carrier infections is greater for crossbreed than native cattle breeds. PLos ONE, 12(3). DOI: 10.1371/journal.pone.0174595
Kumar, T., N. Sindhu, G. Charaya, A. Kumar, P. Kumar and G. Chandratere. 2015. Emerging status of anaplasmosis in cattle in Hisar. Vet. World, 8(6): 768-771. DOI: 10.14202/vetworld.2015.768-771
Kundave, V.R., A.K. Patel, P.V. Patel, J.J. Hasnani and C.G. Joshi. 2015. Detection of theileriosis in cattle and buffaloes by polymerase chain reaction. Journal of Parasitic Diseases, 39(3): 508-513. DOI: 10.1007/s12639-013-0386-2
Lalchandhi, C.L. 2001. Efficacy of various drugs against blood protozoa in Kundhi buffaloes. Parasitologia, 32: 165-176.
Malyar, R.M. and R.A. Farid. 2019. Incidence of haemoprotozoan diseases in cattle & buffaloes Bhisood district Nangarhar province. Journal of Agriculture and Veterinary Science, 12(1): 48-51. Available on: https://www.iosrjournals.org/iosr-javs/papers/Vol12-issue1/Series-3/H1201034851.pdf
Resali, D.P. 2000. Recent trends in buffalo production in Nepal: A review, Buffalo Newsletter. Bulletin of The FAO Inter-Regional Cooperative Research Network on Buffalo, Europe-Near Estate. 14: 6-10.
Yeruham, I., A. Hadani, F. Galker and S. Rosen. 1995. A study of an enzootic focus of sheep babesiosis (Babesia ovis, babes, 1892). Vet. Parasitol., 60(3-4): 349-354. DOI: 10.1016/0304-4017(95)00783-7








.png)








