Development and application of a loop-mediated isothermal amplification method for rapid detection of Pasteurella multocida
DOI:
https://doi.org/10.56825/bufbu.2022.4123763Keywords:
Bubalus bubalis, buffaloes, haemorrhagic septicemia, Pasteurella multocida, kmt1 gene, LAMPAbstract
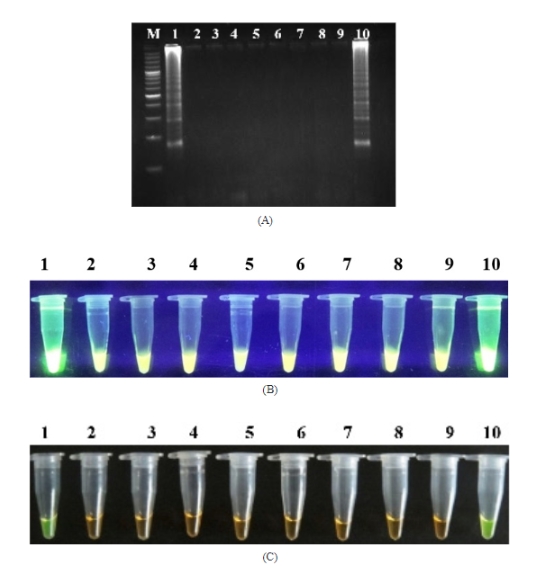
Pasteurella multocida is an important pathogen affecting livestock and poultry causing significant economic losses. A loop-mediated isothermal amplification (LAMP) assay using four primers targeting a conserved region of the kmt1 gene was standardised to diagnose Pasteurella multocida. The test was carried out at 64oC for 45 minutes, the LAMP products could be visually confirmed using fluorescent dye SYBR Green I as detection reagent both with naked eye as well as under UV-illumination. The sensitivity of the developed LAMP assay was 104 fold higher than PCR. Furthermore, no cross-reactivity was found with the other tested bacteria. The developed LAMP assay allows easy, rapid, accurate and sensitive detection of Pasteurella multocida.
Downloads
Metrics
References
Enosawa, M., S. Kageyama, K. Sawai, K. Watanabe, T. Notomi, S. Onoe, Y. Mori and Y. Yokomizo. 2003. Use of loop-mediated isothermal amplification of the IS900 sequence for rapid detection of cultured Mycobacterium avium subsp. paratuberculosis, J. Clin. Microbiol., 41(9): 4359-4365. DOI: 10.1128/JCM.41.9.4359-4365.2003
Hunt, M.L., B. Adler and K.M. Townsend. 2000. The molecular biology of Pasteurella multocida. Vet. Microbiol., 72(1-2): 3-25. DOI: 10.1016/s0378-1135(99)00183-2
Inacio, J., O. Flores and I. Spencer-Martinus. 2008. Efficient identification of clinically relevant Candida yeast species by use of an assay combining panfungal loop-mediated isothermal DNA amplification with hybridization to species-specific oligonucleotide probes. J. Clin. Microbiol., 46(2): 713-720. DOI: 10.1128/JCM.00514-07
Iwamoto, T., T. Sonobe and K. Hayashi. 2003. Loop-mediated isothermal amplification for direct detection of Mycobacterium tuberculosis complex, M. avium, and M. intracellulare in sputum samples, J. Clin. Microbiol., 41(6): 2616-2622. DOI: 10.1128/JCM.41.6.2616-2622.2003
Jabbari, A.R., M. Esmaelzadeh and M.Gh.R. Jula. 2006. Polymerase chain reaction typing of Pasteurella multocida capsules isolated in Iran, Iran. J. Vet. Res., 7(3): 50-55. DOI: 10.22099/IJVR.2006.2649
Maruyama, F., T. Kenzaka, N. Yamaguchi, K. Tani and M. Nasu. 2003. Detection of bacteria carrying the stx2 gene by in situ loop-mediated isothermal amplification, Appl. Environ. Microb., 69(8): 5023-5028. DOI: 10.1128/AEM.69.8.5023-5028.2003
Nagamine, K., T. Hase and T. Notomi. 2002. Accelerated reaction by loop-mediated isothermal amplification using loop primers, Mol. Cell. Probes, 16(3): 223-229. DOI: 10.1006/mcpr.2002.0415
Notomi, T., H. Okayama, H. Masubuchi, T. Yonekawa, K. Watanabe, N. Amino and T. Hase. 2000. Loop-mediated isothermal amplification of DNA, Nucleic Acids. Res., 28(12): e63. DOI: 10.1093/nar/28.12.e63
Parida, M., G. Posadas, S. Inoue, F. Hasebe and K. Morita. 2004. Real-time reverse transcription loop-mediated isothermal amplification for rapid detection of West Nile virus, J. Clin. Microbiol., 42(1): 257. DOI: 10.1128/JCM.42.1.257-263.2004
Savan, R., A. Igarashi, S. Matsuoka and M. Sakai. 2004. Sensitive and rapid detection of edwardsiellosis in fish by a loop-mediated isothermal amplification method, Appl. Environ. Microb., 70(1): 621-624. DOI: 10.1128/AEM.70.1.621-624.2004
Townsend, K.M., A.J. Frost, C.W. Lee, J.M. Papadimitriou and H.J. Dawkins. 1998. Development of PCR assays for species- and type-specific identification of Pasteurella multocida isolates. J. Clin. Microbiol., 36(4): 1096-1100.
Tsai, S.M., K.W. Chan, W.L. Hsu, T.J. Chang, M.L. Wong and C.Y. Wang. 2009. Development of a loop-mediated isothermal amplification for rapid detection of orf virus. J. Virol. Methods., 157(2): 200-204. DOI: 10.1016/j.jviromet.2009.01.003
Wilson, K. 1987. Preparation of genomic DNA from bacteria. In Current Protocols in Molecular Biology, John Wiley and Sons, New York, USA. p. 2.4.1-2.4.2.
Yamazaki, W., M. Ishibashi, R. Kawahara and K. Inoue. 2008. Development of a loop- mediated isothermal amplification assay for sensitive and rapid detection of Vibrio parahaemolyticus. BMC Microbiol., 8. DOI: 10.1186/1471-2180-8-163








.png)








