Quantification of X sperm by raman spectroscopy in percoll density gradient centrifuged buffalo semen
DOI:
https://doi.org/10.56825/bufbu.2023.4243847Keywords:
Bubalus bubalis, buffaloes, Raman spectroscopy, percoll, centrifugation, PBSAbstract
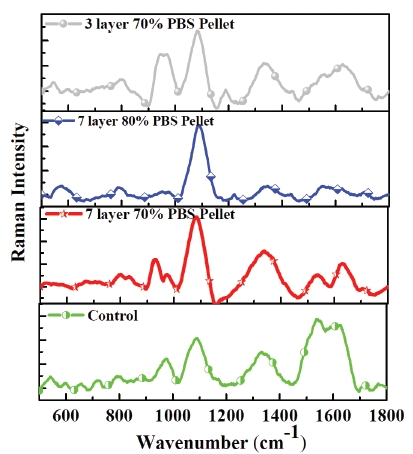
The present study was conducted to observe effect of percoll density gradient centrifugation of buffalo bull semen on quantity of X sperms. Ejaculates were collected by artificial vagina method. Semen with mass motility >+3 and progressive motility >70 % were selected for experiment. X sperm Enrichment of semen was done by discontinuous percoll density gradient centrifugation and three groups were formed ie Group 1 (3 layer 70%, 50% and 30%) Group 2 (7 layer 70%, 60%, 50%, 40%, 30%, 20% and 10%) Group 3 (7 layer 80%, 70%, 60%, 50%, 40%, 30% and 20%). Centrifugation of semen of three groups and control (fresh semen without gradients) was done. After centrifugation, the supernatant part was removed and the pellet of each group was used for X sperm enrichment assessment by Raman spectroscopy. Results revealed that X sperm enrichment was higher in the pellets of Group 2 followed Group 3, Group 1 and Control as Raman peaks on DNA specific bands corresponds to more number of x sperm were higher respectively.
Downloads
Metrics
References
Ahmad, M., S. Rehman, A. Khan and K.M. Ahmad. 1997. Effect of singleand double washing on the liveability of buffalo bullspermatozoa at 37°C. Pak. Vet. J., 17(4): 171-174. Available on: http://www.pvj.com.pk/pdf-files/17_4/171-174.pdf
Andrabi, S.M.H. 2009. Factors affecting the quality of cryopreserved buffalo (Bubalus bubalis) bull spermatozoa. Reprod. Domest. Anim., 44(3): 552-569. DOI: 10.1111/j.1439-0531.2008.01240.x
Baviskar, M.P. 2003. Study of seminal characters and freezing ability of Surti and Murrah buffalo bull semen. M.V.Sc. Thesis, Maharashtra Animal and Fishery Sciences University, Nagpur, India.
Bhakat, M., T.K. Mohanty, S. Singh, A.K. Gupta, A.K. Chakravarty and P. Singh. 2015. Influence of semen collector on semen characteristics of Murrah buffalo and Crossbred bulls. Advances in Animal and Veterinary Sciences, 3(4): 253-258. Available: https://pdfs.semanticscholar.org/2a43/76816cdcc3e9a0270f6b77f00fdf9b82f268.pdf?_gl=1*rik3a5*_ga*MTg0MTA1NzIwNS4xNjk0Njc2MDAy*_ga_H7P4ZT52H5*MTcwMjUyMDYxOC4yMC4wLjE3MDI1MjA2MTkuNTkuMC4w
Bhat, Y. and M. Sharma. 2020. X-sperm enrichment of bovine semen by percoll density gradient method and its effect on semen quality, sex ratio and conception rate. Indian J. Anim. Res., 54(10) 1181-1187. DOI: 10.18805/ijar.B-3823
Blondin, P., M. Beaulieu, V. Fournier, N. Morin, L. Crawford, P. Madan and W.A. King. 2009. Analysis of bovine sexed sperm for IVF from sorting to the embryo. Theriogenology, 71(1): 30-38. DOI: 10.1016/j.theriogenology.2008.09.017
Bodmer, M., F. Janett and M. Hassig. 2005. Fertility in heifers and cows after low dose insemination with sex-sorted and non-sorted sperm under field conditions. Theriogenology, 64(7): 1647-1655. DOI: 10.1016/j.theriogenology.2005.04.011
Carvalho, J.O., R. Sartori, A.P. Lemes, G.B. Mourao and M.A.N. Dode. 2009. Cinética de espermatozoides criopreservados de bovino após sexagem por citometria de fluxoCinética de espermatozoidescriopreservados de bovinoapóssexagemporcitometria de fluxo. Pesqui. Agropecu. Bras., 44(10): p1346-1351. DOI: 10.1590/S0100-204X2009001000019
Cervantes, R.E. and A.C. Izquierdo. 2013. Sexing sperm of domestic animals. Trop. Anim. Health Prod., 45(1): 1-8. DOI: 10.1007/s11250-012-0215-0
Chaudhary, D., K.D. Devlal and M. Sharma. 2022. Study on seminal attributes of X-sperm enriched Sahiwal bull semen. Theriogenology Insight, 12(01): 11-16.
Chaudhary, D., K.D. Devlal and M. Sharma. 2023. Effect of percoll density gradient centrifugation on semen quality of X- sperm enriched crossbred bull semen. Indian J. Anim. Reprod., 4(1): 56-60, DOI: 10.48165/ijar.2023.44.01.11
Chaudhary, D., K.D. Devlal and M. Sharma. 2023. Detection and quantification of X and Y sperms in enriched Sahiwal semen diluted in PBS using Raman spectroscopy. Indian J. Anim. Reprod., 44(2): 46-50.
Cran, D.G., K.H. Lu. and G.E.Jr. Seidel. 1994. In vitro fertilization with flow-cytometrically-sorted bovine sperm. Theriogenology, 52: 1393-1405. DOI: 10.1016/s0093-691x(99)00225-3
Cui, K.H. and C.D. Matthews. 1993. X larger than Y. Nature, 366(6451): 117-118. DOI: 10.1038/366117b0
Cui, K.H. 1997. Size differences between human X and Y spermatozoa and prefertilization diagnosis. Mol. Hum. Reprod., 3(1): 61-67. DOI: 10.1093/molehr/3.1.61
Dash, S. 2017. Contribution of livestock sector to Indian economy. Indian Journal of Research, 6(1): 890-891. Available on: https://www.worldwidejournals.com/paripex/recent_issues_pdf/2017/January/contribution-of-livestock-sector-to-indian-economy_January_2017_1682756615_1515929.pdf
Deka, B.C. and A.R. Rao. 1986. Motility of buck spermatozoa during preservation at 5oC with and without seminal plasma. Indian Vet. J., 63(2): 169-170.
De Jarnette, J.M., R.L. Nebel and C.E. Marshall. 2009. Evaluating the success of sex-sorted semen in US dairy herds from on farm records. Theriogenology, 71(1): 49-58. DOI: 10.1016/j.theriogenology.2008.09.042
De Luca, A.C., S. Managó, M.A. Ferrara, I. Rendina, L. Sirleto, R. Puglisi, D. Balduzzi, A. Galli, P. Ferraro and G. Coppola. 2014. Non-invasive sex assessment in bovine semen by Raman spectroscopy, Laser Phys. Lett., 11(5). DOI: 10.1088/1612-2011/11/5/055604
Galli, A., D. Balduzzi, T. Signori and R. Aleandri. 2009. Evaluation of sperm membrane integrity in frozen buffalo semen. Proceedings of the 18th World Buiatrics Congress, Bologna, Italy.
Garner, D.L., B.L. Gledhill, D. Pinkel, S. Lake, D. Stephenson, M.A. Van Dilla and L.A. Johnson. 1983. Quantification of the X- and Y-chromosome bearing spermatozoa of domestic animals by flow cytometry. Biol. Reprod., 28: 312-321.
Garner, D.L. and L.A. Johnson. 1995. Viability assessment of mammalian sperm using SYBR-14 and propidium iodide. Biol. Reprod., 53(2): 276-284. DOI: 10.1095/biolreprod53.2.276
Hollinshead, F.K., J.K. O’Brien, W.M.C. Maxwell and G. Evans. 2004. Assessment of in vitro sperm characteristics after flow cytometric sorting of frozen-thawed bull spermatozoa. Theriogenology, 62(5): 958-968. DOI: 10.1016/j.theriogenology.2003.12.030
Hossepian De Lima, V.F.M., M.D.T. Ramalho, B.C.A. Alves, A.C. Lucio, L.Z. Oliveira, A.M. Filho and L.C. Carneiro. 2015. Enrichment of bovine semen with X-bearing spermatozoa using Percoll and Optiprep discontinuous gradients, Animal and Veterinary Sciences, 3(1): 1-7. DOI: 10.11648/j.avs.20150301.11
Indian Ministry of Agriculture and Farmer’s Welfare. 2018. National Action Plan for Dairy Development, Government of India, New Delhi, India. 13p.
Johnson, L.A. 2000. Sexing mammalian sperm for production of off spring: The state-of-the-art. Anim. Reprod. Sci., 2(60-61): 93-107. DOI: 10.1016/s0378-4320(00)00088-9
Johnson, L.A. and G.R. Welch. 1999. Sex preselection: High-speed flow cytometric sorting of X and Y sperm for maximum efficiency. Theriogenology, 52(8): 1323-1341. DOI: 10.1016/s0093-691x(99)00220-4
Kiddy, C.A. and H.D. Hafs. 1971. Sex ratio at birth-prospects for control. American Society of Animal Science, 4: 104.
Kishore, S. 1997. Studies on physio-morphology, enzymology and cryopreservation of cross bred bulls, M.V. Sc. Thesis, G.B. Pant University of Agriculture and Technology, Uttarakhand, India. p. 389-403.
Kumar, A. and P. Krupakaran. 2014. Comparative study on physico-morphological characteristics of semen from Murrah buffaloes and Jersey crossbred cattle. CIBTech Journal of Bio-Protocols, 3(3): 12-14.
Lizuka, R., S. Kaneko, K. Kobanawa and T. Kobayashi. 1984. Washing and concentration of human semen by Percoll density gradients and its application to AIH. Andrology Arch. Androl., 20(2): 117-124. DOI: 10.3109/01485018808987061
Lu, K.H., D.G. Cran and G.E. Seidel Jr. 1999. In vitro fertilization with flow-cytometrically sorted bovine sperm. Theriogenology, 52(8): 1393-1405. DOI: 10.1016/s0093-691x(99)00225-3
Lucio, A.C., L.Z. Oliveira and E.C.C. Celeghini. 2008. Influence of bovine subspecies in the recovered rate after the separation of X-bearing sperm by centrifugation in discontinuous Percoll™ density gradient. Anim. Reprod. Sci., 6: 336.
Merton, J.S., R.M. Haring, J. Stap, R.A. Hoebe and J.A. Aten. 1997. Effect of flow cytometrically sorted frozen/thawed semen on success rate of in vitro bovine embryo production. Theriogenology, 47(1): 295.
Miasra, T.P., V.B. Saxena and S.S. Tripathi. 1989. BTrace element in seminal plasma of cross bred bulls. Indian J. Anim. Sci., 59(10): 1245-1248.
Morrell, J.M., K.D. Keeler, D.E. Noakes, N.M. Mackenzie and D.W. Dresser. 1988. Sexing of sperm by flow cytometry. Vet. Rec., 122(14): 322-324. DOI: 10.1136/vr.122.14.322
Moruzzi, J.F. 1979. Selecting a mammalian species for the separation of X- and Y-chromosome-bearing spermatozoa. Reproduction and Fertility, 57(2): 319-323. DOI: 10.1530/jrf.0.0570319
Norman, H.D., J.L. Hutchison and R.H. Miller. 2010. Use of sexed semen and its effect on conception rate, calf sex, dystocia, and stillbirth of Holsteins in the United States. J. Dairy Sci., 93(8): 3880-3890.
Oliveira, L.Z., R.P. Arruda and E.C.C. Celeghini. 2011. Effects of discontinuous prcoll gradient centrifugation on the quality of bovine spermatozoa evaluated with computer-assisted semen analysis and fluorescent probes association. Andrologia, 44(1): 9-15. DOI: 10.1111/j.1439-0272.2010.01096.x
Parrish, J.J., A. Krogenaes and J.L. Susko-Parrish. 1995. Effect of bovine sperm separation by either swim-up or Percoll method on success of in vitro fertilization and early embryonic development. Theriogenology, 44(6): 859-869. DOI: 10.1016/0093-691x(95)00271-9
Patel, K.V. 1985. Diagnostic and investigative andrology in cross bred bulls. Indian J. Anim. Reprod., 6: 107-110.
Prakash, P., L. Leykin and Z. Che. 1998. Preparation by differential gradient centrifugation is better than swim-up in selecting sperm with normal morphology (strict criteria). Fertil. Steril., 69(4): 722-726. DOI: 10.1016/s0015-0282(98)00002-8
Promthep, K., S. Satitmanwiwat, N. Kitiyanant, P. Tantiwattanakul, K. Jirajaroenrat, R. Sitthigripong and C. Singhapol. 2016. Practical use of percoll density gradient centrifugation on sperm sex determination in commercial dairy farm in Thailand. Indian J. Anim. Res., 50(3): 310-313. DOI: 10.18805/ijar.8427
Raizada, B.C., A. Sattar and M.D. Pandey. 1990. A comparative study of freezing buffalo semen in two diluters. In Proceedings of 2nd World Buffalo Congress held in India, Indian Society of Buffalo Development and Indian Council of Agricultural Research, New Delhi, India.
Ramachandran, N. 2000. Studies on fertility performance of Sahiwal bulls. M.Sc. Thesis, National Dairy Research Institute, Karnal, India. 21p.
Resende, M.V., A.C. Lucio, A.P. Perini, L.Z. Oliveira, A.O. Almieda, A.L. Gusmao and V.F.M.H. Lima. 2010. Desvio da proporção de sexo e integridade do DNA dos espermatozoides bovinos centrifugados em gradientes de densidade contínuos. Revista Brasileira de Saúde e Produção Animal, 11: 260-269.
Resende, M.V., A.C. Lucio, A.P. Perini, L.Z. Oliveira, A.O. Almeida, B. da C.A. Alves, C.A. Moreira-Filho, W.F.D. Santos and V.F.M.H. de Lima. 2011. Comparative validation using quantitative real-time PCR (qPCR) and conventional PCR of bovine semen centrifuged in continuous density gradient. Arq. Bras. Med. Vet. Zoo., 63(3): 544-551. DOI: 10.1590/S0102-09352011000300002
Rodriguez-Martinez, H., B. Larsson and H. Pertoft. 1997. Evaluation of sperm damage and techniques for sperm clean-up. Reprod. Fert. Develop., 9: 297-308. DOI: 10.1071/r96081
Saini, A., G. Singh, R.K. Chandolia, R. Dutt and R.K. Malik. 2018. Characteristics of middle uterine artery and fetal umbilical blood flow in pregnant Murrah buffalo. Indian Journal of Animal Reproduction, 39(1): 11-14.
Schenk, J.L., L.S. Herickhoff, S.P. Doyle, Z. Brink, R.D. Green and D.G. Cran. 1999. Insemination of heifers with sexed sperm. Theriogenology, 52: 1407-1420.
Sharma, M., Y. Bhat, N. Sharma and A. Singh. 2018. Comparative study of seasonal variation in semen characteristics of buffalo bull. Journal of Entomology and Zoology Studies, 6(1): 947-951. Available on: https://www.entomoljournal.com/archives/2018/vol6issue1/PartM/6-1-52-109.pdf
Sharma, V., A.K. Verma, P. Sharma, D. Pandey and M. Sharma. 2022. Differential proteomic profile of X- and Y- sorted Sahiwal bull semen. Res. Vet. Sci., 144: 181-189. DOI: 10.1016/j.rvsc.2021.11.013
Sharma, M. and N. Sharma. 2016. Sperm sexing in animals. Advances in Animal and Veterinary Sciences, 4(10): 543- 549. DOI: 10.14737/journal.aavs/2016/4.10.543.549
Sharma, N., D.K. Chand, S. Rawat, M. Sharma and H. Verma. 2018. Effect of sexed semen on conception rate and sex ratio under field conditions. Journal of Entomology and Zoology Studies, 6(1): 702-705. Available on: https://www.entomoljournal.com/archives/2018/vol6issue1/PartJ/6-1-26-860.pdf
Shettles, L.B. 1960. Nuclear morphology of human spermatozoa. Nature, 186: 648-649. DOI: 10.1038/186648a0
Shinde, S.V. 1986. A study on deep- freezing of buffalo bull semen with special reference to addition of caffeine, vitamin-C and PGF2 alpha to the Tris dilutor. M.V. Sc. Thesis, Dr. Balasaheb Sawant Konkan Krishi Vidyapeeth, Maharashtra, India.
Van Kooij, R.J. and B.A. Van Oost. 1992. Determination of sex ratio of spermatozoa with a deoxyribonucleic acid-probe and quinacrine staining: A comparison. Fertil. Steril., 58(2): 384-386. DOI: 10.1016/s0015-0282(16)55226-1
Xu, J., Z. Guo, L. Su, T.L. Nedambale, J. Zhang, J. Schenk, J.F. Moreno, A. Dinnyes, W. Ji, X.C. Tian, X. Yang and F. Du. 2016. Developmental potential of vitrified Holstein cattle embryos fertilized in vitro with sex-sorted sperm. J. Dairy Sci., 89(7): 2510-2518. DOI: 10.3168/jds.S0022-0302(06)72326-8
Zeidan, A.E.B., A.A. El-Zaia, H.E.H. Radwa, J. Rowida, M. Riad and T.A. El-Aasar. 2008. Viability, acrosomal status and sex ratio of the centrifuged rabbit spermatozoa. American-Eurasian Journal of Agricultural and Environmental Sciences, 4(3): 318-325. Available on: https://www.idosi.org/aejaes/jaes4(3)/8.pdf








.png)








