Expression profiling of candidate embryotrophic genes of buffalo oviduct during different stages of oestrous cycle
DOI:
https://doi.org/10.56825/bufbu.2022.4124042Keywords:
Bubalus bubalis, buffaloes, gene expression, buffalo oviduct, oestrous cycle, quantitative PCRAbstract
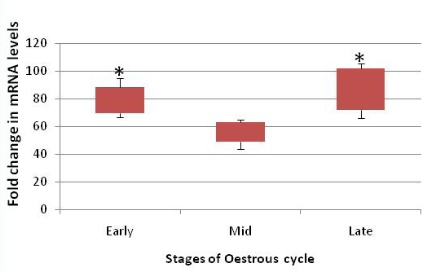
The aim of the present study was to identify the gene expression profile of three candidate genes namely heat shock protein 70, oviductal glycoprotein and osteopontin in buffalo oviduct during different stages of the oestrous cycle. Slaughter house derived buffalo oviducts were categorized as belonging to early, mid and late phase of the oestrous cycle based on the morphological appearance of CL and progesterone concentration. The relative abundance of each of the candidate gene transcript was quantified by real time RT-PCR. Transcripts of all the three candidate genes were found to be expressed in OEC throughout the oestrous cycle. The results of the present study demonstrate that gene expression in the buffalo oviduct is clearly regulated during the oestrous cycle, as these candidate genes play a crucial role in several reproductive processes. It was found that Hsp 70 gene was significantly upregulated in OEC belonging to the early and late phases when compared to mid phases of the cycle. The expression of OVGP mRNA was found to be maximum during the early phase and it remained low during the mid and late phases while expression of OPN mRNA was maximum during the early phase and declined sequentially during the mid and late phase of oestrus cycle.
Downloads
Metrics
References
Bauersachs, S., S. Rehfeld, S.E. Ulbrich, S. Mallok, K. Prelle, H. Wenigerkind, R. Einspanier, H. Blum and E. Wolf. 2004. Monitoring gene expression changes in bovine oviduct epithelial cells during the oestrous cycle. J. Mol. Endocrinol., 32(2): 449- 466. DOI: 10.1677/jme.0.0320449
Bennett, W.A., T.L. Watts, W.D. Blair, S.J. Waldhalam and J.W. Fuquary. 1988. Patterns of oviducal motility in the cow during the oestrous cycle. J. Reprod. Fertil., 83(2): 537-543. DOI: 10.1530/jrf.0.0830537
Boice, M.L., R.D. Geisert, R.M. Blair and H.G. Verhage. 1990. Identification and characterization of bovine oviductal glycoproteins synthesized at oestrus. Biol. Reprod., 43(3): 457-565. DOI: 10.1095/biolreprod43.3.457
Briton-Jones, C., I.H. Lok, C.K. Cheung, T.T.K. Chiu, L.P. Cheung and C. Haines. 2004. Estradiol regulation of oviductin/oviduct-specific glycoprotein messenger ribonucleic acid expression in human oviduct mucosal cells in vitro. Fertil. Steril., 81(1): 749-756. DOI: 10.1016/j.fertnstert.2003.08.016
Buhi, W.C. 1996. Cyclic changes in the oviduct during fertilization and early cleavage-stage embryonic development. Arch. Anim. Breed., 39: 15-25.
Danell, B. 1987. Oestrous behaviour, ovarian morphology and cyclical variation in follicular system and endocrine pattern in Water buffalo heifers. Ph.D. Dissertation, Sveriges Lantbruksuniversitet, Uppsala, Sweden. p. 54-94.
Gabler, C., D.A. Chapman and G.J. Killian. 2003. Expression and presence of osteopontin and integrins in the bovine oviduct during the oestrous cycle. Reproduction, 126(6): 721-729.
Garlow, J.E., H. Ka, G.A. Johnson, R.C. Burghardt, L.A. Jaeger and F.W. Bazer. 2002. Analysis of osteopontin at the maternal-placental interface in pigs. Biol. Reprod., 66(3): 718-725. DOI: 10.1095/biolreprod66.3.718
Goncalves, R.F., C.D. Wolinetz, V.H. Barnabe and G.J. Killian. 2009. Influence of osteopontin in bovine uterine tube fluid on sperm binding and fertilization in RCA-1 lectin-treated oocytes. Reprod. Domest. Anim., 44(1): 152-155. DOI: 10.1111/j.1439-0531.2007.01011.x
Hachen, A.K. Jewgenow and B.C. Braun. 2012. Sequence analysis of feline oviductin and its expression during the estrous cycle in the domestic cat (Felis catus). Theriogenology, 77(3): 539-549. DOI: 10.1016/j.theriogenology.2011.08.029
Hansen, P.J. 2004. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim. Reprod. Sci., 82-83: 349-360. DOI: 10.1016/j.anireprosci.2004.04.011
Ireland, J.J., R.L. Murphee and P.B. Coulson. 1980. Accuracy of predicting stages of bovine estrous cycle by gross appearance of the corpus luteum. J. Dairy Sci., 63: 155-160. DOI: 10.3168/jds.S0022-0302(80)82901-8
Jazayeri, S.P., H. Kohram and R. Salehi. 2010. Hormonal responses to GnRH injection given at different stages of the estrous cycle in water buffaloes. Afr. J. Biotechnol., 9(12): 2169-2172. Available on: https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.993.2784&rep=rep1&type=pdf
Joyce, M.M., J.F. Gonzalez, S. Lewis, S. Woldesenbet, R.C. Burghardt, G.R. Newton and G.A. Johnson. 2005. Caprine uterine and placental osteopontin expression is distinct among epitheliochorial implanting species. Placenta, 26(2-3): 160-170. DOI: 10.1016/j.placenta.2004.05.009
Kenngott, R., M. Vermehren, U. Sauer, K. Ebach and F. Sinowatz. 2011. Cellular expression and localization of estrogen receptor α and progesterone receptor mRNA in the bovine oviduct combining laser-assisted microdissection, quantitative PCR, and in situ hybridization. Journal of Histochemistry and Cytochemistry, 59(3): 312-327.
Killian, G.J., D.A. Chapman, J.F. Kavanaugh, D.R. Deaver and H.B. Wiggin. 1989. Changes in phospholipids, cholesterol and protein content of oviduct fluid of cows during the oestrous cycle. J. Reprod. Fertil., 86(2): 419-426. DOI: 10.1530/jrf.0.0860419
Livak, K.J. and T.D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods, 25(4): 402-408. DOI: 10.1006/meth.2001.1262
Lok, I.H., C.M. Briton-Jones, P.M. Yuen and C.J. Haines. 2002. Variable expression of oviductin mRNA at different stages of human reproductive cycle. J. Assist. Reprod. Gen., 19(12): 569-576. DOI: 10.1023/a:1021263132176
Mariani, M.L., M. Souto, M.A. Fanelli and D.R. Ciocca. 2000. Constitutive expression of heat shock proteins hsp25 and hsp70 in the rat oviduct during neonatal development, the oestrous cycle and early pregnancy. J. Reprod. Fertil., 120(2): 217-223. DOI: 10.1530/jrf.0.1200217
McBride, D.S., C. Boisvert, G. Bleau and F.W. Fan. 2004. Evidence for the regulation of glycosylation of golden hamster (Mesocricetur auratus) oviductin during the estrous cycle. Biol. Reprod., 70(1): 198-203. DOI: 10.1095/biolreprod.103.020305
Meikle, A., E.G. Garófalo, M. Rodríguez-Piñón, C. Tasende and L. Sahlin. 2001. Regulation by gonadal steroids of estrogen and progesterone receptors along the reproductive tract in female lambs. Acta Vet. Scand., 42(1): 161-169. DOI: 10.1186/1751-0147-42-161
Muthukumar, S., R. Rajkumar, K. Karthikeyan, C. Liao, D. Singh, M.A. Akbarsha and G. Archunan. 2014. Buffalo cervico-vaginal fluid proteomics with special reference to estrous cycle: heat shock protein (hsp)-70 appears to be an estrus indicator. Biol. Reprod., 90(5): 1-8. DOI: 10.1095/biolreprod.113.113852
Pradeep, M.A., J. Jagadeesh, A.K. De, J.K. Kaushik, D. Malakar, S. Kumar, A.K. Dang, S.K. Das and A.K. Mohanty. 2011. Purification, sequence characterization and effect of goat oviduct-specific glycoprotein on in vitro embryo development. Theriogenology, 75(6): 1005-1015. DOI: 10.1016/j.theriogenology.2010.11.007
Salvetti, N.R., C. Baravalle, G.A. Mira, E.J. Gimeno, B.E, Dallard, F. Rey and H.H. Ortega. 2008. Heat shock protein 70 and sex steroid receptors in the follicular structures of induced ovarian cysts. Reprod. Domest. Anim., 44(5): 805-814. DOI: 10.1111/j.1439-0531.2008.01086.x
Velazquez, M.A., I. Parrilla, A.V. Soom, S. Verberckmoes, W. Kues and H. Niemann. 2010. Sampling techniques for oviductal and uterine luminal fluid in cattle. Theriogenology, 73(6): 758-767. DOI: 10.1016/j.theriogenology.2009.07.004
Verhage, H.G., P.A. Mavrogianis, M.B. O’Day-Bowman, A. Schmidt, E.B. Arias, K.M. Donnelly, R.A. Boomsma, J.K. Thibodeaux, A.T. Fazleabas and R.C. Jaffe. 1998. Characteristics of an oviductal glycoprotein and its potential role in the fertilization process. Biol. Reprod., 58: 1098-1101. DOI: 10.1095/biolreprod58.5.1098
Wijayagunawardane, M.P.B., W.A. Cerbito and K. Sato. 1996. Oviductal progesterone concentration and its spatial distribution in cyclic and early pregnant cows. Theriogenology, 46(7): 1149-1158. DOI: 10.1016/s0093-691x(96)00286-5








.png)








