Molecular characterization of β-lactoglobulin (βLG) gene in Indian buffalo (Bubalus bubalis)
DOI:
https://doi.org/10.56825/bufbu.2024.4344885Keywords:
Bubalus bubalis, buffaloes, milk, β-lactoglobulin, mammary gland, Epithilial cells, protein structureAbstract
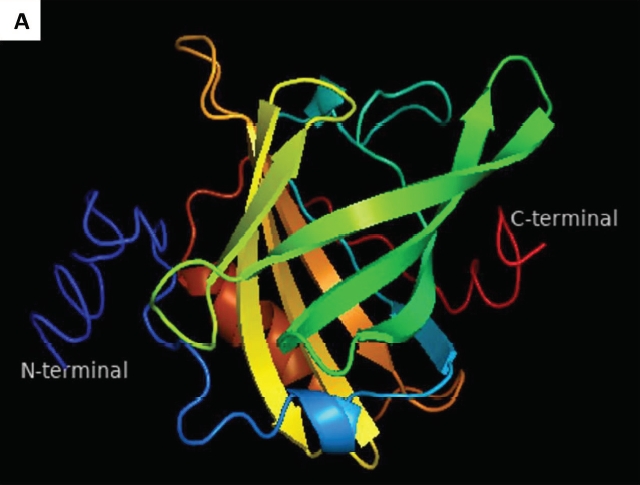
The aim of the study was to characterized the β-lactoglobulin gene and protein in Indian buffalo. The β-lactoglobulin (Bu_βLG) is a major whey milk protein and cause an allergic reaction in infants and children. In the present study, the full open reading frame (ORF) of buffalo β-lactoglobulin (Bu_βLG) gene was characterized, which consisted of a 543 bp sequence with 180 amino acid residues. The phylogenetic tree showed that the cattle, yak, and buffalo βLG gene formed one cluster and buffalo is more closer (96%) to an exotic cow (Bos taurus). ProtParam analysis showed that Bu_βLG protein was acidic (pI, 4.93), thermo-tolerant, and hydrophobic. The Bu_βLG 3-D model was generated by I-TASSER, which revealed a more stabilized nature of the predicted structure. Further, the Ramachandran plot validated that the 3-D model of Bu_βLG protein, which was of good quality. The presence of four ligand-binding sites for retinoic acid, oleic acid, vitamin D3, and benzophenone in Bu_βLG suggests that this protein bind to several fatty acids and ions. IEDB analysis displayed the seven and six epitope sites in the Bu_βLG protein for B-cell and T-cell, respectively. Thus, the epitope sites in the βLG protein may have certain immunological roles, which can be used to reduce the allergenicity of βLG protein for improving the buffalo milk quality.
Downloads
Metrics
References
Aich, R., S. Batabyal and S.N. Joardar. 2014. Simple purification method for beta-lactoglobulin from buffalo milk. Advances in Animal and Veterinary Sciences, 2(2): 78-80. DOI: 10.14737/journal.aavs/2014/2.2.78.80
Anand, V., N. Dogra, S. Singh, S.N. Kumar, M.K. Jena and D. Malakar. 2012. Establishment and characterization of a buffalo (Bubalus bubalis) mammary epithelial cell line. PLoS ONE, 7(7): e40469. DOI: 10.1371/journal.pone.0040469
Aymard, P., D. Durand and T. Nicolaï. 1996. The effect of temperature and ionic strength on the dimerisation of β-lactoglobulin. Int. J. Biol. Macromol., 19(3): 213-221. DOI: 10.1016/0141-8130(96)01130-0
Ball, G., M.J. Shelton, B.J. Walsh, D.J. Hill, C.S. Hosking and M.E. Howden. 1994. A major continuous allergenic epitope of bovine beta-lactoglobulin recognized by human IgE binding. Clin. Exp. Allergy, 24(8): 758-764. DOI: 10.1111/j.1365-2222.1994.tb00987.x
Barłowska, J., A. Wolanciuk, Z. Litwińczuk and J. Król. 2012. Milk proteins’ polymorphism in various species of animals associated with milk production utility, p. 235-264. In Hurley, W.L. (ed.) Milk Protein, Intech Open, USA. DOI: 10.5772/50715
Batra, K., T. Nanda, A. Kumar, R. Kumari, V. Kumar and S. Maan. 2019. Molecular characterization of OAS1 as a biomarker molecule for early pregnancy diagnosis in Bubalus bubalis. Indian J. Biotechnol., 18: 97-107. Available on: https://nopr.niscpr.res.in/bitstream/123456789/49668/3/IJBT%2018(2)%2097-107.pdf
Bawden, W.S., R.J. Passey and A.G. Mackinlay. 1994. The genes encoding the major milk-specific proteins and their use in transgenic studies and protein engineering. Biotechnol. Genet. Eng., 12: 89-138. DOI: 10.1080/02648725.1994.10647910
Berjanskii, M.V. and D.S. Wishart. 2008. Application of the random coil index to studying protein flexibility. J. Biomol. NMR, 40(1): 31-48. DOI 10.1007/s10858-007-9208-0
Brownlow, S., C.J.H. Morais, R. Cooper, D.R. Flower, S.J. Yewdall and I. Polikarpov. 1997. Bovine beta-lactoglobulin at 1.8 A resolution--still an enigmatic lipocalin. Structure, 5(4): 481-495. DOI: 10.1016/s0969-2126(97)00205-0
Chakraborty, J., N. Das, K. Das and U.C. Halder. 2009. Loss of structural integrity and hydrophobic ligand binding capacity of acetylated and succinylated bovine β-lactoglobulin Int. Dairy J., 19(1): 43-49. DOI: 10.1016/j.idairyj.2008.06.011
Creamer, L.K., D.A. Parry and G.N. Malcolm. 1983. Secondary structure of bovine beta-lactoglobulin B. Arch. Biochem. Biophys., 227(1): 98-105. DOI: 10.1016/0003-9861(83)90351-x
Crowther, J.M., G.B. Jameson, A.J. Hodgkinson and R.C.J. Dobson. 2016. Structure, oligomerisation and interactions of β-lactoglobulin. In Gigli, I. (edn.) Milk Proteins - From Structure to Biological Properties and Health Aspects, Intech Open. Argentina. DOI: 10.5772/62992
Crowther, J.M., M. Lassé, H. Suzuki, S.A. Kessans, T.S. Loo and G.E. Norris. 2014. Ultra-high resolution crystal structure of recombinant caprine β-lactoglobulin. FEBS Lett., 588(21): 3816-3822. DOI: 10.1016/j.febslet.2014.09.010
Ding, X., Y. Yang, S. Zhao, Y. Li and Z. Wang. 2011. Analysis of α-lactalbumin, β-lactoglobulin A and B in whey protein powder, colostrum, raw milk, and infant formula by CE and LC. Dairy Sci. Technol., 91(2): 213-225. DOI: 10.1007/s13594-011-0006-9
Filiz, F. and I. Koc. 2014. In silico sequence analysis and homology modeling of predicted beta-amylase 7-like protein in Brachypodium distachyon L. Journal of BioScience and Biotechnology, 3(1): 61-67. Available on: https://core.ac.uk/download/pdf/27172937.pdf
Fogliano, V., S.M. Monti, A. Visconti, G. Randazzo, A.M. Facchiano and G. Colonna. 1998. Identification of a beta-lactoglobulin lactosylation site. Biochim. Biophys. Acta, 1388(2): 295-304. DOI: 10.1016/s0167-4838(98)00177-0
Gasteiger, E., A. Gattiker, C. Hoogland, I. Ivanyi, R.D. Appel and A. Bairoch. 2003. ExPASy: The proteomics server for in-depth protein knowledge and analysis. Nucleic Acids Res., 31(13): 3784-3788. DOI: 10.1093/nar/gkg563
Geourjon, C. and G. Deleage. 1995. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Comput. Appl. Biosci., 11(6): 681-684. DOI: 10.1093/bioinformatics/11.6.681
Haug, A., A.T. Høstmark and O.M. Harstad. 2007. Bovine milk in human nutrition - A review. Lipids Health Dis., 6: 25. DOI: 10.1186/1476-511X-6-25
Hoffman, J.R. and M.J. Falvo. 2004. Protein - Which is best? J. Sport. Sci. Med., 3(3): 118-130.
Inoue, R., S. Matsushita, H. Kaneko, S. Shinoda, H. Sakaguchi and Y. Nishimura. 2001. Identification of beta-lactoglobulin-derived peptides and class II HLA molecules recognized by T cells from patients with milk allergy. Clin. Exp. Allergy, 31(7): 1126-1134. DOI: 10.1046/j.1365-2222.2001.01135.x
Islam, M.A., M.K. Alam, M.N. Islam, M.A. Khan, D. Ekeberg and E.O. Rukke. 2014. Principal milk components in buffalo, Holstein cross, indigenous cattle and red Chittagong cattle from Bangladesh. Asian-Austral. J. Anim., 27(6): 886-897. DOI: 10.5713/ajas.2013.13586
Jameson, G.B., J.J. Adams and L.K. Creamer. 2002. Flexibility, functionality and hydrophobicity of bovine β-lactoglobulins. Int. Dairy J., 12(4): 319-329. DOI: 10.1016/S0958-6946(02)00028-6
Kontopidis, G., A.N. Gilliver and L. Sawyer. 2014. Ovine β-lactoglobulin at atomic resolution. Acta Crystallographica Section F: Structural Biology Communications, 70(11): 1498-1503. DOI: 10.1107/S2053230X14020950
Kontopidis, G., C. Holt and L. Sawyer. 2002. The ligand-binding site of bovine beta-lactoglobulin: Evidence for a function?. J. Mol. Biol., 318(4): 1043-1055. DOI: 10.1016/S0022-2836(02)00017-7
Kontopidis, G., C. Holt and L. Sawyer. 2004. Invited review: beta-lactoglobulin: binding properties, structure, and function. J. Dairy Sci., 87(4): 785-96. DOI: 10.3168/jds.S0022-0302(04)73222-1
Kumar, S., C.J. Tsai and R. Nussinov. 2000. Factors enhancing protein thermostability. Protein Eng. Des. Sel., 13(3): 179-191. DOI: /10.1093/protein/13.3.179
Kuwata, K., M. Hoshino, S. Era, C.A. Batt and Y. Goto. 1998. Α to β transition of β-lactoglobulin as evidenced by heteronuclear NMR. J. Mol. Biol., 283(4): 731-739. DOI: 10.1006/jmbi.1998.2117
Larsen, J.E., O. Lund and M. Nielsen. 2006. Improved method for predicting linear B-cell epitopes. Immunome Research, 2: 2. DOI: 10.1186/1745-7580-2-2
Laskowski, R.A., M.W. MacArthur, D.S. Moss and J.M. Thornton. 1993. PROCHECK: A program to check the stereochemical quality of protein structures, J. Appl. Crystallogr., 26: 283-291. DOI: 10.1107/S0021889892009944
Le Maux, S., S. Bouhallab, L. Giblin, A. Brodkorb and T. Croguennec. 2014. Bovine β-lactoglobulin/fatty acid complexes: binding, structural, and biological properties. Dairy Sci. Technol., 94(5): 409‐426. DOI: 10.1007/s13594-014-0160-y
Liang, L., H.A. Tajmirriahi and M. Subirade. 2008. Interaction of beta-lactoglobulin with resveratrol and its biological implications. Biomacromolecules, 9(1): 50-56. DOI: 10.1021/bm700728k
Loch, J.I., P. Bonarek, A. Polit, D. Riès, M. Dziedzicka-Wasylewska and K. Lewiński. 2013. Binding of 18-carbon unsaturated fatty acids to bovine β-lactoglobulin-structural and thermodynamic studies. Int. J. Macromol., 57: 226-231. DOI: 10.1016/j.ijbiomac.2013.03.021
Loch, J.I., M. Molenda, M. Kopeć, S. Świątek and K. Lewiński. 2014. Structure of two crystal forms of sheep β-lactoglobulin with EF‐loop in closed conformation. Biopolymers, 101(8): 886-894. DOI: 10.1002/bip.22471
Lönnerdal, B. 2003. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr., 77(6): 1537S-1543S. DOI: 10.1093/ajcn/77.6.1537S
Lönnerdal, B. 2004. Human milk proteins: Key components for the biological activity of human milk. Adv. Exp. Med. Biol., 554: 11-25. DOI: 10.1007/978-1-4757-4242-8_4
Luhovyy, B.L., T. Akhavan and G.H. Anderson. 2007. Whey proteins in the regulation of food intake and satiety. J. Am. Coll. Nutr., 26(6): 704S-712S. DOI: 10.1080/07315724.2007.10719651
Mercadante, D., L.D. Melton, G.E. Norris, T.S. Loo, M.A.K. Williams, R.C.J. Dobson and G.B. Jameson. 2012. Bovine β-lactoglobulin is dimeric under imitative physiological conditions: dissociation equilibrium and rate constants over the pH range of 2.5-7.5. Biophys. J., 103(2): 303-312. DOI: 10.1016/j.bpj.2012.05.041
Niemi, M., S. Jylhä, M.L. Laukkanen, H. Söderlund, S.M. Kiljunen, J.M. Kallio, N. Hakulinen, T. Haahtela, K. Takkinen and J. Rouvinen. 2007. Molecular interactions between a recombinant IgE antibody and the beta-lactoglobulin allergen. Structure, 15(11): 1413-21. DOI: 10.1016/j.str.2007.09.012
Otaviano, A.R., A.L.F. Lima, M.M.M. Laureano, J.A.D. Sena, L.G. and H. Tonhati. 2008. β-casein gene polymorphism permits identification of bovine milk mixed with bubaline milk in mozzarella cheese. Genet. Mol. Biol., 31(4): 902-905. DOI: 10.1590/S1415-47572008005000002
Papiz, M.Z., L. Sawyer, E.E. Eliopoulos, A.C. North, J.B. Findlay, R. Sivaprasadarao, T.A. Jones, M.E. Newcomer and P.J. Kraulis. 1986. The structure of beta-lactoglobulin and its similarity to plasma retinol-binding protein. Nature, 324(6095): 383-385. DOI: 10.1038/324383a0
Paul, S., C.S.L. Arlehamn, T.J. Scriba, M.B.C. Dillon, C. Oseroff, D. Hinz, D.M. McKinney, S.C. Pro, J. Sidney, B. Peters and A. Sette. 2014. Development and validation of a broad scheme for prediction of HLA class II restricted T cell epitopes. J. Immunol. Methods, 422: 28-34. DOI: 10.1016/j.jim.2015.03.022
Pérez, M.D. and M. Calvo. 1995. Interaction of beta-lactoglobulin with retinol and fatty acids and its role as a possible biological function for this protein: A review. J. Dairy Sci., 78(5): 978-988. DOI: 10.3168/jds.S0022-0302(95)76713-3
Picariello, G., M. De Cicco, R. Nocerino, L. Paparo, G. Mamone, F. Addeo and R.B. Canani. 2019. Excretion of dietary cow’s milk derived peptides into breast milk. Frontiers in Nutrition, 6: 25. DOI: 10.3389/fnut.2019.00025
Puyol, P., M.D. Perez, L. Mata, J.M. Ena and M. Calvo. 1993. Effect of retinol and fatty acid binding by bovine β-lactoglobulin on its resistance to trypsin digestion. Int. Dairy J., 3(7): 589-597. DOI: 10.1016/0958-6946(93)90102-6
Renard, D., J. Lefebvre, M.C.A. Griffin and W.G. Griffin. 1998. Effects of pH and salt environment on the association of beta-lactoglobulin revealed by intrinsic fluorescence studies. Int. J. Biol. Macromol., 22(1): 41-49. DOI: 10.1016/s0141-8130(97)00086-x
Roth-Walter, F., L.F. Pacios, C.G. Casado, G. Hofstetter, G.A. Roth, J. Singer, A.D. Perales and E.J. Jarolim. 2014. The major cow milk allergen bos d 5 manipulates t-helper cells depending on its load with siderophore-bound iron. PLoS ONE, 9(8): e104803 DOI: 10.1371/journal.pone.0104803
Sawyer, L. and G. Kontopidis. 2000. The core lipocalin, bovine beta-lactoglobulin. Biochim. Biophys. Acta, 1482(1-2): 136-148. DOI: 10.1016/s0167-4838(00)00160-6
Sélo, I., G. Clément, H. Bernard, J. Chatel, C. Créminon, G. Peltre and J. Wal. 1999. Allergy to bovine beta-lactoglobulin: Specificity of human IgE to tryptic peptides. Clinical & Experimental Allergy, 29(8): 1055-1063. DOI: 10.1046/j.1365-2222.1999.00612.x
Song, C.Y., W.L. Chen, M.C. Yang, J.P. Huang, and S.J.T. Mao. 2005. Epitope mapping of a monoclonal antibody specific to bovine dry milk: involvement of residues 66-76 of strand D in thermal denatured beta-lactoglobulin. J. Biol. Chem., 280(5): 3574-3582. DOI: 10.1074/jbc.M407031200
Totsuka, M., A. Ametani and S. Kaminogawa. 1997. Fine mapping of T-cell determinants of bovine beta-lactoglobulin. Cytotechnology, 25(1-3): 101-113. DOI: 10.1023/a:1007967901271
Verheul, M., J.S. Pedersen, S.P. Roefs and K.G. de Kruif. 1999. Association behavior of native beta-lactoglobulin. Biopolymers, 49(1): 11-20. DOI: 10.1002/(SICI)1097-0282(199901)49:1<11::AID-BIP2>3.0.CO;2-1
Waterhouse, A., M. Bertoni, S. Bienert, G. Studer, G. Tauriello, R. Gumienny, F.T. Heer, T.A.P. de Beer, C. Rempfer, L. Bordoli, R. Lepore and T. Schwede. 2018. SWISS-MODEL: Homology modelling of protein structures and complexes, Nucleic Acids Research, 46: 296-303. DOI: 10.1093/nar/gky427
Zhang, Y. 2008. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics, 9: 40. DOI: 10.1186/1471-2105-9-40








.png)








