The milk IGF-2 level is positively correlated with milk yield in Anatolian water buffaloes
DOI:
https://doi.org/10.56825/bufbu.2023.4225257Keywords:
Bubabus bubalis, buffaloes, Anatolian water buffalo, insulin-like growth factor-2, milk yieldAbstract
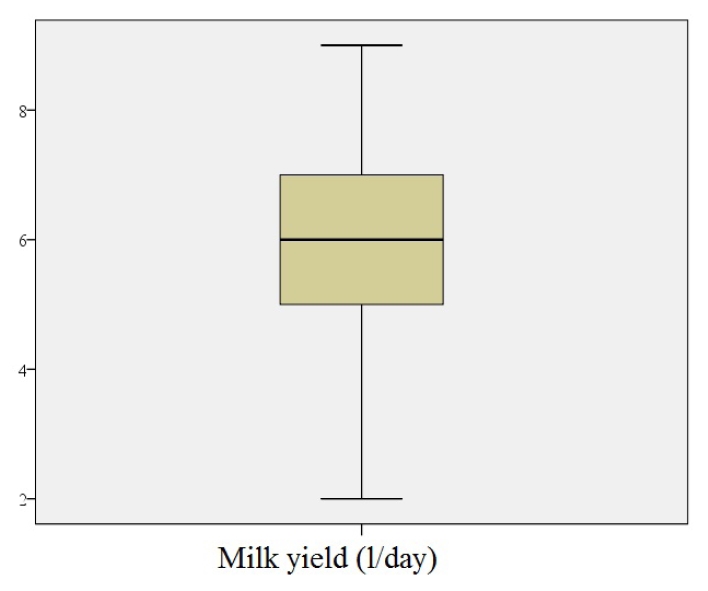
The aim of this study was to investigate the relationship between milk insulin-like growth factor-2 (IGF-2) concentration and milk yield in Anatolian water buffaloes. This study was conducted on milk samples from 80 Anatolian water buffaloes. Milk samples collected from buffaloes were evaluated for subclinical mastitis. For this purpose, the California mastitis test and somatic cell count were performed in milk samples taken from four different mammary lobes of buffaloes. The milk IGF-2 level was determined by an enzyme-linked immunosorbent assay kit. Milk IGF-2 concentration ranged from 21.6 ng/ml - 63.2 ng/ml in Anatolian water buffaloes. The IGF-2 concentration in Anatolian water buffaloes milk was 40.1±8.5 ng/ml. Milk IGF-2 was positively correlated with milk yield (r2=0.941, P<0.01). Our results showed that milk IGF-2 concentration was associated with milk yield in Anatolian buffaloes. These findings show that locally synthesized IGF-2 can affect milk yield. This study contributes to the understanding of the composition of buffalo milk, which has an important value in human nutrition. It is recommended to confirm the results of similar measurements in milk from other animal species used for human consumption.
Downloads
Metrics
References
Arrichiello, A., G. Auriemma and F. Sarubbi. 2022. Comparison of nutritional value of different ruminant milks in human nutrition. International Journal of Functional Nutrition, 3(4): 1-10. DOI: 10.3892/ijfn.2022.28
Bagnicka, E., E. Siadkowska, N. Strzałkowska, B. Zelazowska, K. Flisikowski, J. Krzyzewski and L. Zwierzchowski. 2010. Association of polymorphisms in exons 2 and 10 of the insulin-like growth factor 2 (IGF2) gene with milk production traits in Polish Holstein-Friesian cattle. J. Dairy Res., 1: 37-42. DOI: 10.1017/S0022029909990197
Baker, J., J.P. Liu, E.J. Robertson and A. Efstratiadis. 1993 Role of insulin-like growth factors in embryonic and postnatal growth. Cell, 75(1): 73-82. DOI: 10.1016/S0092-8674(05)80085-6
Bonakdar, E., H.R. Rahmani, M.A. Edriss and B.E.S. Tabatabaei. 2010. IGF-I gene polymorphism, but not its blood concentration, is associated with milk fat and protein in Holstein dairy cows. Genet. Mol. Res., 9(3): 1726-1734. DOI: 10.4238/vol9-3gmr874
Brisken, C., A. Ayyannan, C. Nguyen, A. Heineman, F. Reinhardt, J. Tan, S.K. Dey, G.P. Dotto and R.A. Weinberg. 2002. IGF-2 is a mediator of prolactin-induced morphogenesis in the breast. Dev. Cell, 3(6): 877-887. DOI: 10.1016/S1534-5807(02)00365-9
Colarow, L., M. Turini, S. Teneberg and A. Berger. 2003. Characterization and biological activity of gangliosides in buffalo milk. Biochim. Biophys. Acta, 1631(1): 94-106. DOI: 10.1016/S1388-1981(02)00360-8
De Matteis, G., F. Grandoni, M. Zampieri, A. Reale and M.C. Scatà. 2021. New insights into the significance of parp-1 activation: flow cytometric detection of poly (ADP-Ribose) as a marker of bovine intramammary infection. Cells, 10(3): 599. DOI: 10.3390/cells10030599
D’Onofrio, N., A. Balestrieri, G. Neglia, A. Monaco, M. Tatullo, R. Casale, A. Limone, M.L. Balestrieri and G. Campanile. 2019. Antioxidant and anti-inflammatory activities of buffalo milk δ-Valerobetaine. J. Agr. Food Chem., 67(6): 1702-1710. DOI: 10.1021/acs.jafc.8b07166
Elmlinger, M.W., F. Hochhaus, A. Loui, K.W. Frommer, M. Obladen and M.B. Ranke. 2007. Insulin-like growth factors and binding proteins in early milk from mothers of preterm and term infants. Horm. Res., 68(3): 124-131. DOI: 10.1159/000100488
El-Moghazy, M.M., M.N.F. Hamad and A.A. El-Raghi. 2015. The relation between milk yield and composition of individual buffaloes. Egyptian Journal of Dairy Science, 43: 141-145.
Faienza, M.F., N. Santoro, R. Lauciello, R. Calabrò, L. Giordani, G. Di Salvo, A. Ventura, M. Delvecchio, L. Perrone, E.M. Del Giudice and L. Cavallo. 2010. IGF2 gene variants and risk of hypertension in obese children and adolescents. Pediatr. Res., 67(4): 340-344. DOI: 10.1203/PDR.0b013e3181d22757
Fiedler, J., C. Brill, W.F. Blum and R.E. Brenner. 2006. IGF-I and IGF-II stimulate directed cell migration of bone-marrow-derived human mesenchymal progenitor cells. Biochem. Bioph. Res. Co., 345(3): 1177-1183. DOI: 10.1016/j.bbrc.2006.05.034
Forsyth, I.A., G. Gabai and G. Morgan. 1999. Spatial and temporal expression of insulin-like growth factor-I, insulin-like growth factor-II and the insulin-like growth factor-I receptor in the sheep fetal mammary gland. J. Dairy Res., 66(1): 35-44. DOI: 10.1017/S0022029998003240
Goelz, R., E. Hihn, K. Hamprecht, K. Dietz, G. Jahn, C. Poets and M. Elmlinger. 2009. Effects of different CMV-heat-inactivation-methods on growth factors in human breast milk. Pediatr. Res., 65(4): 458-461. DOI: 10.1203/PDR.0b013e3181991f18
Grochowska, R., P. Sørensen, L. Zwierzchowski, M. Snochowski and P. Løvendahl. 2001. Genetic variation in stimulated GH release and in IGF-I of young dairy cattle and their associations with the leucine/valine polymorphism in the GH gene. J. Anim. Sci., 79: 470-476. DOI: 10.2527/2001.792470x
Hovey, R.C., J. Harris, D.L. Hadsell, A.V. Lee, C.J. Ormandy and B.K. Vonderhaar. 2003. Local insulin-like growth factor-II mediates prolactin-induced mammary gland development. Mol. Endocrinol., 17(3): 460-471. DOI: 10.1210/me.2002-0214
Kang, S.H., J.U. Kim, Y. Kim, K.S. Han, W.J. Lee, J.Y. Imm and S.H. Kim. 2007. Changes in the levels of insulin-like growth factors (IGF-I and IGF-II) in bovine milk according to the lactation period and parity. Asian-Austral. J. Anim. Sci., 20(1): 119-123. DOI: 10.5713/ajas.2007.119
Kilicoglu, C., E. Alacam, H. Izgur, O. Akay and H.U. Wiesner. 1989. Eutergesundheitskontrolle von Milchkülen im Gebiet von Ankara (Turkei). Deut. Tierärztl. Woch., 96: 486-488.
Li, Z., S. Lu, K. Cui, L. Shafique, S.U. Rehman, C. Luo, Z. Wang, J. Ruan, Q. Qian and Q. Liu 2020. Fatty acid biosynthesis and transcriptional regulation of Stearoyl-CoA Desaturase 1 (SCD1) in buffalo milk. BMC Genet., 21(1): 23. DOI: 10.1186/s12863-020-0829-6
Milsom, S.R., W.F. Blum and A.J. Gunn. 2008. Temporal changes in insulin-like growth factors I and II and in insulin-like growth factor binding proteins 1, 2, and 3 in human milk. Horm. Res., 69(5): 307-311. DOI: 10.1159/000114863
Morali, O.G., A. Jouneau, K.J. McLaughlin, J.P. Thiery and L. Larue. 2000. IGF-II promotes mesoderm formation. Dev. Biol., 227(1): 133-145. DOI: 10.1006/dbio.2000.9875
Nielsen, F.C. 1992. The molecular and cellular biology of insulin-like growth factor II. Progress in Growth Factor Research, 4(3): 257-290. DOI: 10.1016/0955-2235(92)90023-B
Nosho, K., H. Yamamoto, H. Taniguchi, Y. Adachi, Y. Yoshida, Y. Arimura, T. Endo, Y. Hinoda and K. Imai. 2004. Interplay of insulin-like growth factor-II, insulin-like growth factor-I, insulin-like growth factor-I receptor, COX-2, and matrix metalloproteinase-7, play key roles in the early stage of colorectal carcinogenesis. Clin. Cancer Res., 10(23): 7950-7957. DOI: 10.1158/1078-0432.CCR-04-0875
Piecewicz, S.M., A. Pandey, B. Roy, S.H. Xiang, B.R. Zetter and S. Sengupta. 2012. Insulin-like growth factors promote vasculogenesis in embryonic stem cells. PLOS One, 7(2): e32191. DOI: 10.1371/journal.pone.0032191
Plath-Gabler, A., C. Gabler, F. Sinowatz, B. Berisha and D. Schams. 2001. The expression of the IGF family and GH receptor in the bovine mammary gland. J. Endocrinol., 168(1): 39-48. DOI: 10.1677/joe.0.1680039
Prosser, C.G., S.R. Davis, V.C. Farr, L.G. Moore and P.D. Gluckman. 1994. Effects of close-arterial (external pudic) infusion of insulin-like growth factor-II on milk yield and mammary blood flow in lactating goats. J. Endocrinol., 142(1): 93-99. DOI: 10.1677/joe.0.1420093
Raj, A., V. Kulangara, T.P. Vareed, D.P. Melepat, L. Chattothayil and S. Chullipparambil. 2021. Variations in the levels of acute-phase proteins and lactoferrin in serum and milk during bovine subclinical mastitis. J. Dairy Res., 88(3): 321-325. DOI: 10.1017/S002202992100056X
Richardson, A.E., N. Hamilton, W. Davis, C. Brito and D. De León. 2011. Insulin-like growth factor-2 (IGF-2) activates estrogen receptor-α and -β via the IGF-1 and the insulin receptors in breast cancer cells. Growth Factors, 29(2-3): 82-93. DOI: 10.3109/08977194.2011.565003
Ron, M., G. Israeli, E. Seroussi, J.I. Weller, J.P. Gregg, M. Shani and J.F. Medrano. 2007. Combining mouse mammary gland gene expression and comparative mapping for the identification of candidate genes for QTL of milk production traits in cattle. BMC Genomics, 8(1): 183. DOI: 10.1186/1471-2164-8-183
Roth, S.M., M.A. Schrager, E.J. Metter, S.E. Riechman, J.L. Fleg, B.F. Hurley and R.E. Ferrell 2002. IGF2 genotype and obesity in men and women across the adult age span. Int. J. Obesity, 26(4): 585-587. DOI: 10.1038/sj.ijo.0801927
Sadek, K., E. Saleh and M. Ayoub. 2017. Selective, reliable blood and milk bio-markers for diagnosing clinical and subclinical bovine mastitis. Trop. Anim. Health Pro., 49(2): 431-437. DOI: 10.1007/s11250-016-1190-7
Salman, M., M. Khaskheli, I.U. Haq, A.R. Talpur, A.P. Khuhro, M. Rauf, H. Hamid and A. Aziz. 2014. Comparative studies on nutritive quality of buffalo and cow milk. International Journal of Research in Applied, Natural and Social Sciences, 2(12): 69-78.
Schalm, O.W., E.J. Carrol and N.C. Jain. 1971. Bovine Mastitis, Lea-Febiger, Phildelphia, USA. p. 94-124.
Servillo, L., N. D’Onofrio, G. Neglia, R. Casale, D. Cautela, M. Marrelli, A. Limone, G. Campanile and M.L. Balestrieri. 2018. Carnitine precursors and short-chain acylcarnitines in water buffalo milk. Journal of Agricultural and Food Chemistry, 66: 8142-8149. DOI: 10.1021/acs.jafc.8b02963
Simões da Silva, T.M., A.C. Meirelles Piazentin, C.M. Nóbrega Mendonça, A. Converti, C.S. Bittencourt Bogsan, D. Mora and R.P. de Souza Oliveira. 2020. Buffalo milk increases viability and resistance of probiotic bacteria in dairy beverages under in vitro simulated gastrointestinal conditions. J. Dairy Sci., 103(9): 7890-7897. DOI: 10.3168/jds.2019-18078
Simões, P.B.A, M. Campbell, L. Viora, J. Gibbons, T.E. Geraghty, P.D. Eckersall and R.N. Zadoks. 2018. Pilot study into milk haptoglobin as an indicator of udder health in heifers after calving. Res. Vet. Sci., 116: 83-87. DOI: 10.1016/j.rvsc.2017.05.024
Spicer, L.J. and P.Y. Aad 2007. Insulin-like growth factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine granulosa cells through the IGF1 receptor: role of follicle-stimulating hormone and IGF2 receptor. Biol. Reprod., 77(1): 18-27. DOI: 10.1095/biolreprod.106.058230
Szewczuk, M., M. Bajurna, S. Zych and W. Kruszyński. 2013. Association of insulin-like growth factor I gene polymorphisms (IGF1/TasI and IGF1/SnaBI) with the growth and subsequent milk yield of Polish Holstein-Friesian heifers. Czech J. Anim. Sci., 58(9): 404-411. DOI: 10.17221/6940-CJAS
Tatullo, M., B. Marrelli, C. Benincasa, E. Aiello, M. Amantea, S. Gentile, N. Leonardi, M.L. Balestrieri and G. Campanile. 2022. Potential impact of functional biomolecules-enriched foods on human health: A randomized controlled clinical trial. Int. J. Med. Sci., 19(3): 563-571. DOI: 10.7150/ijms.70435
Taylor, V.J., Z. Cheng, P.G. Pushpakumara, D.E. Beever and D.C. Wathes. 2004. Relationships between the plasma concentrations of insulin-like growth factor-I in dairy cows and their fertility and milk yield. Vet. Rec., 155(19): 583-588. DOI: 10.1136/vr.155.19.583
Tripaldi, C. and G. Palocci. 2008. Milk composition and quality. Bulletin of the International Dairy Federation, 426: 11-15.
Wang, C., X. Li, H. Dang, P. Liu, B.O. Zhang and F. Xu. 2019. Insulin-like growth factor 2 regulates the proliferation and differentiation of rat adipose-derived stromal cells via IGF-1R and IR. Cytotherapy, 21(6): 619-630. DOI: 10.1016/j.jcyt.2018.11.010
Wilson, E.M. and P. Rotwein. 2006. Control of MyoD function during initiation of muscle differentiation by an autocrine signaling pathway activated by insulin-like growth factor-II. Journal of Biological Chemistry, 281(40): 29962-29971. DOI: 10.1074/jbc.M605445200








.png)








