Comparative efficacy of coconut water diluent with different semen extenders for cryopreservation of Nili Ravi buffalo bull semen
DOI:
https://doi.org/10.56825/bufbu.2022.4132994Keywords:
Bubalus bubalis, buffaloes, spermatozoa, semen, cryopreservation, extenderAbstract
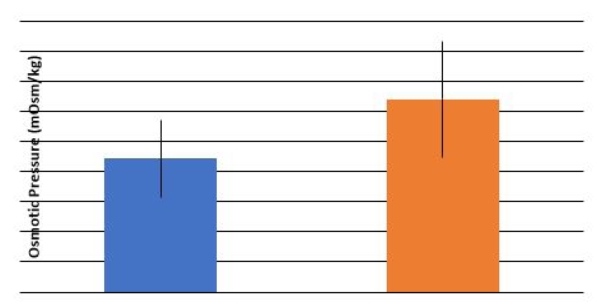
In this study, efforts were made to investigate fresh semen parameters and to select a suitable extender for buffalo semen cryopreservation. Experiment I, fresh undiluted seminal parameters were determined while Experiment II, efficacy comparison of coconut water extender (CWE) with tris citric acid extender (TCAE) and skimmed milk extender (SME) was made. Four bulls single ejaculate was collected weekly for 5 and 10 weeks for Experiment I and II, respectively however osmotic pressure replicates were 16 and 6 for semen and seminal plasma, respectively. In Experiment I, each bull spermatozoa concentration, motility (%), semen volume and pooled semen percentage NAR, PMI, viability and MTT (3-(4, 5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide) reduction rate was checked. In Experiment II, pooled semen added to extenders and then equilibrated (4oC) and filled to obtain 20×106 spermatozoa/0.5 ml straws before plunging in liquid nitrogen. Percentage thawed spermatozoa viability, normal acrosomal ridge (NAR), motility, DNA damage, plasma membrane integrity (PMI) and lipid peroxidation (nM) recorded. In Experiment I, seminal parameters as spermatozoa concentration (1226.43±71.48 million/mL), semen volume (2.84±0.14 mL), viability (90.05±0.71%), motility (77.13±0.71%), PMI (86.23±0.34%), NAR (94.67±0.30%) and MTT reduction rate (0.290±0.06) while osmotic pressure of seminal plasma (294.83±3.87 mOsm/kg) and semen (290.87±2.58 mOsm/kg) was recorded. In Experiment II, TCAE higher (P<0.05) sperm motility noted compared to CWE and SME whereas percentage viability, NAR, PMI, DNA damage was non-significant. Lipid peroxidation compared to SME and TCAE was higher (P<0.05) in CWE. In conclusion, based on sperm motility and lipid per oxidation, TCAE was more efficient for cryopreservation of buffalo semen.
Downloads
Metrics
References
Aguiar, P.H.P., V.J. Andrade, J.J. Abreu and N.B.N. Gomez. 1994. Physical and morphological semen characteristics of buffaloes aged from four to eight years old. In Proceedings of the 4th World Buffalo Congress, Sao Paulo, Brazil. 3: 486-488.
Aitken, R.J. and C. Krausz. 2001. Oxidative stress, DNA damage and the Y chromosome. Reproduction, 122: 497-506.
Akhtar, S., M.S. Ansari, B.A. Rakha. S.M.H. Andrabi, S. Iqbal and N. Ullah. 2010. Cryopreservation of buffalo Bubalus bubalis semen in Bioxcell extender. Theriogenology, 74(6): 951-955. DOI: 10.1016/j.theriogenology.2010.04.024
Akhtar, T. and R.A. Chaudry. 1989. Effect of different extenders on extracellular release of hyaluronidase from buffalo bull semen. Buffalo J., 2: 137-142.
Andrabi, S.M.H., M.S. Ansari, N. Ullah, M. Anwar, A. Mehmood and S. Akhter. 2008. Duck egg yolk in extender improves the freezability of buffalo bull spermatozoa. Anim. Reprod. Sci., 104(2-4): 427-433. DOI: 10.1016/j.anireprosci.2007.07.003
Anonymous. 2010-2011. Economic Survey of Pakistan 2010-2011. Agricultural Census Organization, Statistics Division, Government of Pakistan, Islamabad, Pakistan. p. 15-33.
Ansari, M.S., B.A. Rakha, N. Ullah, S.M.H. Andrabi, S. Iqbal, M. Khalid and S. Akhter. 2010. Effect of exogenous glutathione in extender on the freezability of Nili-Ravi buffalo (Bubalus bubalis) bull spermatozoa. Anim. Sci. Pap. Rep., 28(3): 235-244. Available on: http://archiwum.ighz.edu.pl/files/objects/7507/66/str_235-244.pdf
Ardon, F., D. Helms, E. Sahin, H. Bollwein, E. Topfer-Petersen and D. Waberski. 2008. Chromatin-unstable boar spermatozoa have little chance of reaching oocytes in vivo. Reproduction, 135(4): 461-470. DOI: 10.1530/REP-07-0333
Bilal, M.Q., M. Suleman and A. Raziq. 2006. Buffalo: black gold of Pakistan. Livestock Research for Rural Development, 18(9): 9-15.
Budworth, P.R., R.P. Amann and P.L. Chapman. 1988. Relationships between computerized measurements of motion of frozen-thawed bull spermatozoa and fertility. J. Androl., 9(1): 41-54. DOI: 10.1002/j.1939-4640.1988.tb01007.x
Cardoso, R.C.S., A.R. Silva, D.C. Uchao and L.D.M. Silva. 2003. Cryopreservation of canine semen using a coconut water extender with egg yolk and three different glycerol concentrations. Theriogenology, 59(3-4): 743-751. DOI: 10.1016/s0093-691x(02)01151-2
Chaudhari, A.R., P. Das and R. Singh. 2008. Study of oxidative stress and reduced glutathione levels in seminal plasma of human subjects with different fertility potential. Biomed. Res. India, 19(3): 207-210.
Cordova, A., J.F. Perez-Gutierrez, B. Lleo, C. Garcıa-Artiga, A. Alvarez, V. Drobchak and R.S. Martın. 2002. In vitro fertilizing capacity and chromatin condensation of deep frozen boar semen packaged in 0.5 and 5 ml straws. Theriogenology, 57(8): 2119-2128. DOI: 10.1016/s0093-691x(02)00701-x
El-Sisy, G.A., R.I. El-Sheshtawy, A.A. Mohamed and W.S. El-Nattat. 2010. Correlations between semen parameters and conception rate in buffaloes. Global Veterinaria, 5(1): 15-20. Available on: https://www.idosi.org/gv/gv5(1)10/4.pdf
Fiser, P.S. and R.W. Fairfull. 1986. Combined effects of glycerol concentration, cooling velocity and osmolality of skim milk diluents on cryopreservation of ram spermatozoa. Theriogenology, 25(3): 473-484. DOI: 10.1016/0093-691x(86)90057-9
Fiser, P.S. and R.W. Fairfull. 1989. The effect of glycerol related osmotic changes on post-thaw motility and acrosomal integrity of ram spermatozoa. Cryobiology, 26(1): 64-69. DOI: 10.1016/0011-2240(89)90033-3
Fiser, P.S., L. Ainsworth and R.W. Fairfull. 1982. Cryosurvival of ram spermatozoa in hypertonic and isotonic diluents. Can. J. Anim. Sci., 62(2): 425-428. DOI: 10.4141/cjas82-049
Fiser, P.S., L. Ainsworth and G.A. Langford. 1981. Effect of osmolality of skim-milk diluents and thawing rate on cryosurvival of ram spermatozoa. Cryobiology, 18(4): 399-403. DOI: 10.1016/0011-2240(81)90113-9
Foote, R.H., Y. Chen and C.C. Brockett. 1993. Fertility of bull spermatozoa frozen in whole milk extender with trehalose, taurine or blood serum. J. Dairy Sci., 76(7): 1908-1913. DOI: 10.3168/jds.S0022-0302(93)77524-4
Glogowski, J., J. Jazdzewski and J. Strzezek. 1994. Intensity of 3H-actinomycin D (3H-AMD) binding to chromatin of bull spermatozoa. Reprod. Domest. Anim., 29: 396-403. DOI: 10.1111/j.1439-0531.1994.tb00586.x
Good, N.E., G.D. Winget, W. Winter, T.N. Connolly, S. Izawa and R.M.M. Singh. 1966. Hydrogen ion buffers for biological research. Biochemistry, 5(2): 467-472. DOI: 10.1021/bi00866a011
Hashemi, A., P. Farhoomand, R. Pirmohammadi, S. Razzaghzadeh and M. Nayebpor. 2007. Effect of extender on sperm motility and acrosomal integrity of frozen buffalo sperm. J. Anim. Vet. Adv., 6(11): 1340-1342. Available on: http://docsdrive.com/pdfs/medwelljournals/javaa/2007/1340-1342.pdf
Ibrahim, S., S.A.I. El-Azab, A.M. Racka and F.A. Soliman. 1985. The physico-chemical characteristics of the pre-ejaculate fraction, whole semen and the seminal plasma in buffalo bulls. In Proceeding 1st World Buffalo Congress, Cairo, Egypt. 1: 1042-1051.
Ijaz, A., A. Hussain, M. Aleem, M.S. Yousaf and H. Rehman. 2009. Butylated hydroxytoluene inclusion in semen extender improves the post-thawed semen quality of Nili-Ravi buffalo (Bubalus bubalis). Theriogenology, 71(8): 1326-1329. DOI: 10.1016/j.theriogenology.2008.12.023
Iqbal, M., M. Aleem, A. Ijaz, H. Rehman and M.S. Yousaf. 2010. Assessment of buffalo semen with the 3-4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium bromide reduction assay. J. Anim. Sci., 88(3): 922-925. DOI: 10.2527/jas.2009-2344
Kanwal, M.R., N.U. Rehman, N. Ahmad, H.A. Samad, Z.U. Rehman, N. Akhtar and S. Ali. 2000. Bulk cations and trace elements in the Nili-Ravi buffalo and crossbred cow bull semen. Int. J. Agric. Biol., 2(4): 302-305. Available on: http://www.fspublishers.org/published_papers/55312_..pdf
Khan, M.I.R. and A. Ijaz. 2008. Effects of osmotic pressure on motility, plasma membrane integrity and viability in fresh and frozen-thawed buffalo spermatozoa. Animal, 2(4): 548-553. DOI: 10.1017/S1751731108001596
Kommisrud, E., T. Graffer and T. Steine. 1996. Comparison of two processing systems for bull semen with regard to post-thaw motility and nonreturn rates. Theriogenology, 45(8): 1515-1521. DOI: 10.1016/0093-691X(96)00119-7
Kumar, R., G.J.M. Arvind and S.K. Atreja. 2011. Freeze-thaw induced genotoxicity in buffalo (Bubalus bubalis) spermatozoa in relation to total antioxidant status. Mol. Biol. Rep., 38(3): 1499-1506. DOI: 10.1007/s11033-010-0257-1
Lewis, S.E.M. and R.J. Aitken. 2005. DNA damage to spermatozoa has impacts on fertilization and pregnancy. Cell Tissue Res., 322(1): 33-41. DOI: 10.1007/s00441-005-1097-5
Liete, T.G., V.R.V. Filho, R.P. Arruda, A.F.C. Andrade, L.L. Emerick, F.G. Zaffalon, J.A.M. Martins and V.J. Andrade. 2010. Effects of extender and equilibration time on post-thaw motility and membrane integrity of cryopreserved Gyr bull semen evaluated by CASA and flow cytometry. Anim. Reprod. Sci., 120(1-4): 31-38. DOI: 10.1016/j.anireprosci.2010.04.005
Mann, T. 1964. The Biochemistry of Semen and Male Reproductive Tract, 2nd ed. Metheun Press, London, UK. p. 265-364.Meyers, S.A. 2005. Spermatozoal response to osmotic stress. Anim. Reprod. Sci., 89(1-4): 57-64. DOI: 10.1016/j.anireprosci.2005.06.026
Mughal, D.H., A. Ijaz, M.S. Yousaf, H. Rehman, M. Aleem, H. Zaneb and F. Wadood. 2013. Assessment of optimal osmotic pressure of citrate egg yolk extender for cryopreservation of buffalo bull (Bubalus bubalis) semen. J. Anim. Plant Sci., 23(4): 964-968. Available on: http://thejaps.org.pk/docs/v-23-4/03.pdf
Nur, Z., B. Zik, B. Ustuner, H. Sagirkaya and C.G. Ozguden. 2010. Effects of different cryoprotective agents on ram sperm morphology and DNA integrity. Theriogenology, 73(9): 1267-1275. DOI: 10.1016/j.theriogenology.2009.12.007
Ohkawa, H., H. Kawa, N. Ohishi and K. Yagi. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem., 95: 35l-358. DOI: 10.1016/0003-2697(79)90738-3
Pommer, A.C., J. Ruttlant and S.A. Meyers. 2002. The role of osmotic resistance on equine spermatozoa function. Theriogenology, 58(7): 1373-1384. DOI: 10.1016/s0093-691x(02)01039-7
Presicce, G. 2007. Reproduction in the Water buffalo. Reprod. Domest. Anim., 42(2): 24-32. DOI: 10.1111/j.1439-0531.2007.00907.x
Raizada, B.C., A. Sattar and M.D. Pandey. 1990. A comparative study of freezing buffalo semen in two diluters. In Proceedings of 2nd World Buffalo Congress Held in India, Indian Society of Buffalo Development and Indian Council of Agricultural Research, New Delhi, India.
Rasul, Z., M. Anzar, S. Jalali and N. Ahmad. 2000. Effect of buffering systems on post-thaw motion characteristics, plasma membrane integrity, and acrosome morphology of buffalo spermatozoa. Anim. Reprod. Sci., 59(1-2): 31-41. DOI: 10.1016/s0378-4320(00)00070-1
Rasul, Z., N. Ahmad and M. Anzar. 2001. Changes in motion characteristics, plasma membrane integrity and acrosome morphology during cryopreservation of buffalo spermatozoa. J. Androl., 22(2): 278-283. DOI: 10.1002/j.1939-4640.2001.tb02181.x
Royere, D., S. Hamamah, J.C. Nicolle and J. Lansac. 1991. Chromatin alterations induced by freezing-thawing influence the fertilizing ability of human sperm. Int. J. Androl., 14(5): 328-332. DOI: 10.1111/j.1365-2605.1991.tb01100.x
Saacke, R.G. and J.M. White. 1972. Semen quality tests and their relationship to fertility. In Proceeding 4th NAAB Technical Conference on Artificial Insemination and Reproduction, Madison, Wisconsin. National Association of Animal Breeders, Columbia, Missouri, USA. p. 22-27.
Sakkas, D. and J.G. Alvarez. 2010. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil. Steril., 93(4): 1027-1036. DOI: 10.1016/j.fertnstert.2009.10.046
Salamon, S. and W.M. Maxwell. 2000. Storage of ram semen. Anim. Reprod. Sci., 62(1-3): 77-111. DOI: 10.1016/s0378-4320(00)00155-x
Singh, L.P., M.H. Harshan and M.R. Ansari. 2007. Effect of egg yolk and seminal plasma heparin binding protein interaction on the freezability of buffalo cauda epididymal spermatozoa. Anim. Reprod. Sci., 99(3-4): 395-400. DOI: 10.1016/j.anireprosci.2006.08.018
Songsasen, N., I. Yu, S. Murton, D.L. Paccamonti, B.E. Eilts, R.A. Godke and R.A. Leibo. 2002. Osmotic sensitivity of canine spermatozoa. Cryobiology, 44(1): 79-90. DOI: 10.1016/S0011-2240(02)00009-3
Tejada, R.I., J.C. Mitchell, A. Norman, J.J. Marik and S. Friedman. 1984. A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertil. Steril., 42(1): 87-91. DOI: 10.1016/s0015-0282(16)47963-x
Tuli, R.K., M. Singh and J.S. Matharoo. 1981. Effect of different equilibration times and extenders on deep freezing of buffalo semen. Theriogenology, 16(1): 99-104. DOI: 10.1016/0093-691x(81)90118-7
Wadood, F., M. Aleem, A. Ijaz, N. Ahmad, M.S. Yousaf and D.H. Mughal. 2016. Effect of extender osmolality and butylated hydroxy toluene supplementation on post thaw quality and fertility of nili ravi buffalo bull (Bubalus bubalis) semen. J. Anim. Plant Sci., 26(3): 605-611.
Waterhouse, K.E., A. Gjeldnes, A. Tverdal, P.M.D. Angelis, W. Farstad, M. Haard and E. Kommisrud. 2010. Alterations of sperm DNA integrity during cryopreservation procedure and in vitro incubation of bull semen. Anim. Reprod. Sci., 117: 34-42. DOI: 10.1016/j.anireprosci.2009.04.011
Watson, P.F. 1979. The preservation of semen in mammals. In Finn, C.A. (ed.) Oxford Reviews of Reproductive Biology, Oxford University Press, Dondon, UK. 1: 283-350.
Watson, P.F. 2000. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci., 2(60-61): 481-492. DOI: 10.1016/s0378-4320(00)00099-3
Woelders, H., A. Matthijs and B. Engel. 1997. Effects of trehalose and sucrose, osmolality of the freezing medium, and cooling rate on viability and intactness of bull sperm after freezing and thawing. Cryobiology, 35(2): 93-105. DOI: 10.1006/cryo.1997.2028
Yildiz, C., P. Ottaviani, N. Law, R. Yearst, L. Liu and C. Mckerlie. 2007. Effects of cryopreservation on sperm quality, nuclear DNA integrity, in vitro fertilization, and in vitro embryo development in the mouse. Reproduction, 133(3): 585-595. DOI: 10.1530/REP-06-0256








.png)








