Peptidoglycan recognition protein-1 gene polymorphism and its association with mastitis in Murrah buffalo
DOI:
https://doi.org/10.56825/bufbu.2024.4323666Keywords:
Bubalus bubalis, buffaloes, bovine, mastitis, peptidoglycan recognition protein-1, polymorphismAbstract
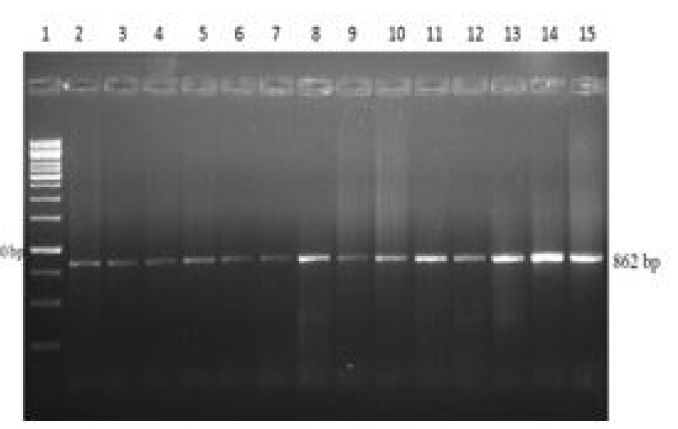
A wide variety of environmental or contagious microorganisms implicated in mastitis, impede the economic growth of dairy sector. Identification of polymorphism in candidate gene of host’s immune system and to rule out mastitis resistant allelic form of candidate gene usually remains prime focal point of research. Bovine peptidoglycan recognition protein-1 (PGLYRP-1), exclusively present in the granules of polymorphonuclear leukocytes has direct microbicidal properties. The present study was carried out to find the association between PGLYRP-1 polymorphic alleles with mastitis. Milk samples for somatic cell count and blood samples for PCR-RFLP analysis of PGLYRP-1 gene were collected from 20 mastitis negative and 20 mastitis positive Murrah buffaloes. There was significant difference in somatic cell count of mastitis and mastitis free animals. All amplified PCR products of ~862 bp size of partial region of PGLYRP-1 gene were subjected to each restriction enzyme (HincII or TaqαΙ or ApaI). Polymorphism in the partial region of PGLYRP-1 gene had not been established using PCR-RFLP as uniformity in pattern of digested fragments was seen. Target sequence PGLYRP-1 gene of Murrah buffalo was cloned and sequenced. BLAST analysis revealed sequence identity of PGLYRP-1 of Murrah buffalo with Bos taurus (JN085441.1) sequence at NCBI was 96%, 96% with Bos indicus (JN085440.1) and 96% with Bos indicus X Bos taurus (EU746454.1). In phylogenetic tree, the target sequence of PGLYRP-1 gene of Bubalus bubalis are found more closely related to Bos taurus than to Bos indicus.
Downloads
Metrics
References
Agrawal, A., R. Varshney, P. Kirthika, R. Gupta, S. Sulabh, R. Varshney, S. Chakravarti and S. Thankappan. 2019. Global scenario of paratuberculosis: a threat to livestock sector. Biol. Rhythm Res., 52(6): 957-972 DOI: 10.1080/09291016.2019.1610858
Alluwaimi, A.M., C.M. Leutenegger, T.B. Farver, P.V. Rossitto, W.L. Smith and J.S. Cullor. 2003. The cytokine markers in Staphylococcus aureus mastitis of bovine mammary gland. J. Vet. Med., 50(3): 105-111. DOI: 10.1046/j.1439-0450.2003.00628.x
Bhattacharya, S.S., A.F. Wright, J.F. Clayton, W.H. Price, C.I. Phillips, C.M.E. Mckeown, M. Jay, A.C. Bird, P.L. Pearson, E.M. Southern and H.J. Evans. 1984. Close genetic linkage between X-linked retinitis pigmentosa and a restriction fragment length polymorphism identified by recombinant DNA probe L1.28. Nature, 309(5965): 253-255. DOI: 10.1038/309253a0
Bytyqi, H., U. Zaugg, K. Sherifi, A. Hamidi, M. Gjonbalaj, S. Muji and H. Mehmeti. 2010. Influence of management and physiological factors on somatic cell count in raw milk in Kosova. Vet. Arhiv., 80(2): 173-183. Available on: https://hrcak.srce.hr/file/85438
Detilleux, J.C., K.J. Koehler, A.E. Freeman, M.E. Kehrli and D.H. Kelley. 1994. Immunological parameters of periparturient Holstein cattle: Genetic variation. International Journal of Dairy Science, 77(9): 2640-2650. DOI: 10.3168/jds.S0022-0302(94)77205-2
Gelius, E., C. Persson, J. Karlsson and H. Steiner. 2003. A mammalian peptidoglycan recognition protein with N-acetylmuramoyl-L-alanine amidase activity. Biochem. Bioph. Res. Co., 306(4): 988-994. DOI: 10.1016/s0006-291x(03)01096-9
Gusella, J.F., N.S. Wexler, P.M. Conneally, S.L. Nayor, M.A. Anderson, A.B. Young, I. Shoulson, E. Bonilla, J.B. Martin. 1983. A polymorphic DNA marker genetically linked to Huntingon’s disease. Nature, 306: 234-238.
Ivinson, A.J. and G.R. Taylor. 1992. PCR in genetic diagnosis, p. 15-28. In McPherson, P., G.R. Quirke and M.J. Taylor. PCR- A Practical Approach (eds.) Oxford University Press, Wellington, UK.
Jain, A.K. 2009. Analysis of factors affecting milk production trait of Murrah buffalo over different lactations. Indian J. Dairy Sci., 62(5): 381-385.
Kang, D., G. Liu, A. Lundstrom, E. Gelius and H. Steiner. 1998. A peptidoglycan recognition protein in innate immunity conserved from insects to mammal. P. Natl. Acad. Sci. USA., 95(17): 10078-10082. DOI: 10.1073/pnas.95.17.10078
Little, P.F.R., S.G. Annison, R. Darling, L. Williamson, B. Camba and Modell. 1980. Model for antenatal diagnosis of beta-thalassaemia and other monogenic disorders by molecular analysis of linked DNA polymorphisms. Nature, 285(5761): 144-147. DOI: 10.1038/285144a0
Liu, C., Z. Xu, D. Gupta and R. Dziarski. 2001. Peptidoglycan recognition proteins: A novel family of four human innate immunity pattern recognition molecules. J. Biol. Chem., 276(37): 34686-34694. DOI: 10.1074/jbc.M105566200
Liu, W., Y.F. Yao, L. Zhou, Q.Y. Ni and H.L. Xu. 2013. Evolutionary analysis of the short-type peptidoglycan-recognition protein gene (PGLYRP1) in primates. Genet. Mol. Res., 12(1): 453-62. DOI: 10.4238/2013.February.8.10a
Murray, J.M., K.E. Davies, P.S. Harper, L. Meredith, C.R. Mueller and R. Williamson. 1982. Linkage relationship of a cloned DNA sequence on the short arm of the X chromosome to Duchenne muscular dystrophy. Nature, 300(5887): 69-71. DOI: 10.1038/300069a0
Ogorevc, J., T. Kunej, A. Razpet and P. Dovc. 2009. Database of cattle candidate genes and genetic markers for milk production and mastitis. Anim. Genet., 40(6): 832-851. DOI: 10.1111/j.1365-2052.2009.01921.x
Oltenacu, P.A. and D.M. Broom. 2010. The impact of genetic selection for increased milk yield on the welfare of dairy cows. Anim. Welfare, 19(1): 39-49. DOI: 10.1017/S0962728600002220
Oviedo-Boyso, J., J.J. Valdez-Alarcón, M.Cajero-Juárez, A. Ochoa-Zarzosa and J.E. López-Meza. 2007. Innate immune response of bovine mammary gland to pathogenic bacteria responsible for mastitis. J. Infection, 54(4): 399-409. DOI: 10.1016/j.jinf.2006.06.010
Pant, S.D., C.P. Verschoor, F.S. Schenkel, Q. You, D.F. Kelton and N.A. Karrow. 2011. Bovine PGLYRP1 polymorphisms and their association with resistance to Mycobacterium avium ssp. paratuberculosis. Anim. Genet, 42(4): 354-60. DOI: 10.1111/j.1365-2052.2010.02153.x
Rambeaud, M., R.A. Almeida, G.M. Pighetti and S.P. Oliver. 2003. Dynamics of leukocytes and cytokines during experimentally induced Streptococcus uberis mastitis. Vet. Immunol. Immunop., 96(3-4): 193-205. DOI: 10.1016/j.vetimm.2003.08.008
Sambrook, J., E.F. Fritsch and T. Maniatis.1989. Molecular Cloning- A Laboratory Manual. Cold Spring Harbor Laboratory Press, New York, USA. p. 6.3-6.4.
Sambrook, J. and D.W. Russel. 2001. Molecular Cloning: A Laboratory Manual, 3rd ed. Cold Spring Harbar Lab. Press, New York, USA.
Sulabh, S., M. Panigrahi, S.F. Ahmad, R. Varshney, A. Verma, N.A. Baba, S. Kumar, S. Kumari, A. Chauhan, P. Kumar and B. Bhushan. 2018. Peptidoglycan and lipoteichoic acid induces differential mRNA response of immune-related genes in PBMC of crossbred, Tharparkar cattle and Murrah buffalo. Anim. Biotechnol., 30(2): 166-174. DOI: 10.1080/10495398.2018.1461633
Sulabh, S., M. Panigrahi, S. Kumar, R. Varshney, A. Verma, N.A. Baba, J.P. Gupta, A. Chauhan, P. Kumar, T. Dutt and B. Bhushan. 2019. Differential cytokine response of Escherichia coli lipopolysaccharide stimulated peripheral blood mononuclear cells in crossbred cattle, Tharparkar cattle and Murrah buffalo - An in vitro study. Span. J. Agric Res., 17(1): e0501. DOI: 10.5424/sjar/2019171-12599
Tydell, C.C., J. Yuan, P. Tran and M.E. Selsted. 2006. Bovine peptidoglycan recognition protein-S: Antimicrobial activity, localization, secretion, and binding properties. J. Immunol., 176(2): 1154-1162. DOI: 10.4049/jimmunol.176.2.1154








.png)








