GENISTEIN DECREASES THE NITRIC OXIDE INDUCED ACROSOME REACTION BY INHIBITING TYROSINE PHOSPHORYLATION IN MURRAH BUFFALO SPERMATOZOA
DOI:
https://doi.org/10.56825/bufbu.2025.4414469Keywords:
Bubalus bubalis, buffaloes, Spermine-NONOate, acrosome reaction, protein Tyrosine phosphorylation, genisteinAbstract
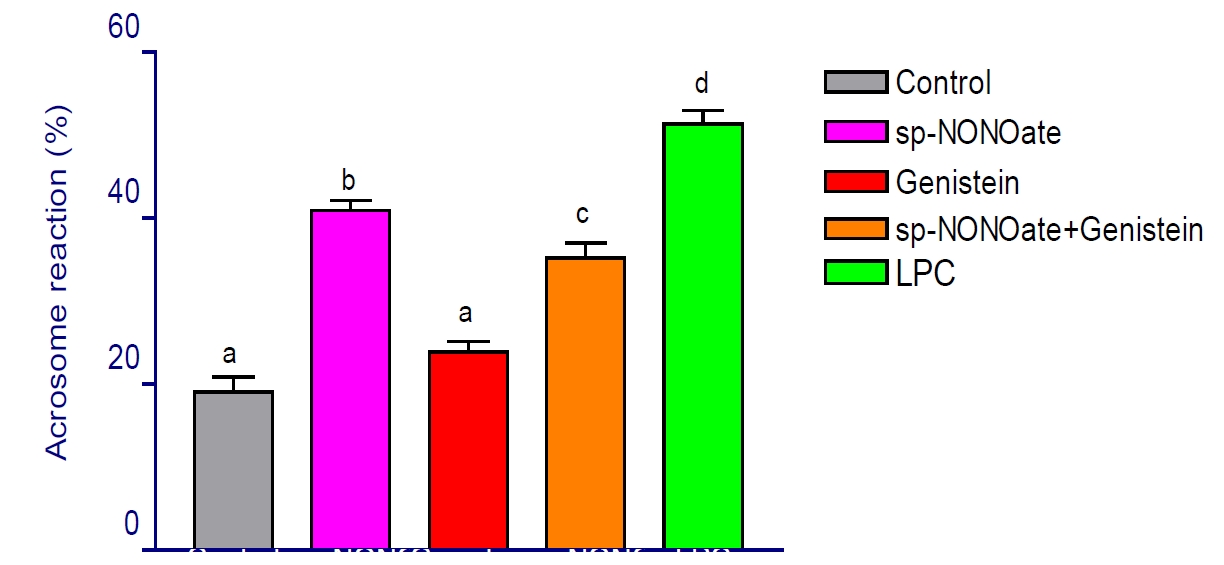
Spermine-NONOate, a nitric oxide donor, contributes to various physiological functions, including the acrosome reaction (AR) at physiological levels. It triggers AR by enhancing tyrosine phosphorylation in proteins ranging from 20 to 105 kDa. Genistein, an isoflavonoid known to inhibit protein tyrosine kinase, significantly (P<0.05) reduces the AR percentage compared to Spermine-NONOate. Furthermore, LPC alone markedly increases the AR percentage (P<0.05) relative to the control (51.36±1.03% vs. 19.09±1.38%). Spermine-NONOate treatment elevates phosphorylation in proteins p20, p30, p38, p80, and p105, but this phosphorylation is significantly decreased (P<0.05) when genistein is present. Notably, p20 and p30 show higher phosphorylation in the Spermine-NONOate group but are absent in both the genistein-only and Spermine-NONOate+genistein groups, with p30 specifically undetectable after genistein treatment. In contrast, proteins p80 and p105 experience substantial tyrosine phosphorylation in the Spermine-NONOate group, which diminishes significantly (P<0.05) with genistein. This decrease in tyrosine phosphorylation during AR in the presence of genistein suggests its inhibitory effect on nitric oxide-induced AR, indicating that nitric oxide facilitates AR in buffalo spermatozoa through protein tyrosine kinase-dependent phosphorylation.
Downloads
Metrics
References
Agarwal, A., K.P. Nallella, S.S. Allamaneni and T.M. Said. 2004. Role of antioxidants in treatment of male infertility: An overview of the literature. Reprod. Biomed. Online, 8(6): 616-627. DOI: 10.1016/s1472-6483(10)61641-0
Aitken, R.J., M. Paterson, H. Fisher, D.W. Buckingham and M. van Duin. 1995. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J. Cell Sci., 108: 2017-2025. DOI: 10.1242/jcs.108.5.2017
Akiyama, T., J. Ishida, S. Nakagawa, H. Ogawara, S. Watanabe and N. Itoh. 1987. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem., 262(12): 5592-5595. Available on: https://www.jbc.org/article/S0021-9258(18)45614-1/pdf
Bajpai, M. and G. Doncel. 2003. Involvement of tyrosine kinase and cAMP-dependent kinase cross-talk in the regulation of human sperm motility. Reproduction, 126(2): 183-195. DOI: 10.1530/rep.0.1260183
Baldi, E., R. Casano, C. Falsetti, C.S. Krausz, M. Maggi and G. Forti. 1991. Intracellular calcium accumulation and responsiveness to progesterone in capacitating human spermatozoa. J. Androl., 12(5): 323-330. DOI: 10.1002/j.1939-4640.1991.tb01610.x
Bauskin, A.R., I. Alkalay and Y. Ben-Neriah. 1991. Redox regulation of a protein tyrosine kinase in the endoplasmic reticulum. Cell, 66(4): 685-696. DOI: 10.1016/0092-8674(91)90114-e
De Lamirande, E. and C. Gagnon. 2002. The extracellular signal-regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion. Mol. Hum. Reprod., 8(2): 124-135. DOI: 10.1093/molehr/8.2.124
De Lamirande, E., A. Harakat and C. Gagnon. 1998. Human sperm capacitation induced by biological fluids and progesterone, but not by NADH or NADPH, is associated with the production of superoxide anion. J. Androl., 19(2): 215-225. DOI: 10.1002/j.1939-4640.1998.tb01991.x
Dixon, R.A. 2004. Phytoestrogens. Annu. Rev. Plant Biol., 55: 225-261. DOI: 10.1146/annurev.arplant.55.031903.141729
Galantino-Homer, H.L., P.E. Visconti and G.S. Kopf. 1997. Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3’5’-monophosphate-dependent pathway. Biol. Reprod., 56(3): 707-719. DOI: 10.1095/biolreprod56.3.707
Ganai, A.A. and H. Farooqi. 2015. Bioactivity of genistein: A review of in vitro and in vivo studies. Biomed. Pharmacother., 76: 30-38. DOI: 10.1016/j.biopha.2015.10.026
Guzman-Grenfell, A.M., S.R. Hernandez, M.T. Gonzalez-Martinez and J.J. Hicks. 1999. Effect of nitric oxide releasers on some metabolic processes of rabbit spermatozoa. Arch. Andrology, 42(2): 119-123. DOI: 10.1080/014850199262968
Herrero, M.B., J.M. Viggiano, S.P. Martinez and M.E. Gimeno. 1997. Evidence that nitric oxide synthase is involved in progesterone-induced acrosomal exocytosis in mouse spermatozoa. Reprod. Fert. Develop., 9(4): 433-439. DOI: 10.1071/r96044
Herrero, M.B., E. De Lamirande and C. Gagnon. 1999. Nitric oxide regulates human sperm capacitation and protein-tyrosine phosphorylation in vitro. Biol. Reprod., 61(3): 575-581. DOI: 10.1095/biolreprod61.3.575
Joo, B.S., S.H. Park, S.J. Park, H.S. Kang, H.S. Moon and H.D. Kim. 1999. The effect of nitric oxide on sperm cell function and embryo development. Am. J. Reprod. Immunol., 42(6): 327-334. DOI: 10.1111/j.1600-0897.1999.tb00109.x
Kim, S.H., C.W. Kim, S.Y. Jeon, R.E. Go, K.A. Hwang and K.C. Choi. 2014. Chemopreventive and chemotherapeutic effects of genistein, a soy isoflavone, upon cancer development and progression in preclinical animal models. Laboratory Animal Research, 30(4): 143-150. DOI: 10.5625/lar.2014.30.4.143
Kirkman-Brown, J.C., L. Lefievre, C. Bray, P.M. Stewart, C.L. Barratt and S.J. Publicover. 2002. Inhibitors of receptor tyrosine kinases do not suppress progesterone-induced [Ca2+]i signalling in human spermatozoa. Mol. Hum. Reprod., 8(4): 326-332. DOI: 10.1093/molehr/8.4.326
Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227: 680-685. DOI: 10.1038/227680a0
Leclerc, P., E. De Lamirande and C. Gagnon. 1996. Cyclic adenosine 3’,5’monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol. Reprod., 55(3): 684-695. DOI: 10.1095/biolreprod55.3.684
Leclerc, P., E. de Lamirande and C. Gagnon. 1997. Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radical Bio. Med., 22(4): 643-656. DOI: 10.1016/s0891-5849(96)00379-6
Lewis, S.E.M., E.T. Donnelly, E.S.L. Sterling, M.S. Kennedy, W. Thompson and U. Chakravarthy. 1996. Nitric oxide synthase and nitrite production in human spermatozoa: evidence that endogenous nitric oxide is beneficial to sperm motility. Mol. Hum. Reprod., 2(11): 873-878. DOI: 10.1093/molehr/2.11.873
Leyton, L. and P. Saling. 1989. 95 kd sperm proteins bind ZP3 and serve as tyrosine kinase substrates in response to zona binding. Cell, 57(7): 1123-1130. DOI: 10.1016/0092-8674(89)90049-4
Leyton, L., P. LeGuen, D. Bunch and P.M. Saling. 1992. Regulation of mouse gamete interaction by a sperm tyrosine kinase. P. Natl. Acad. Sci. USA, 89(24): 11692-11695. DOI: 10.1073/pnas.89.24.11692
Luconi, M., L. Bonaccorsi, C. Krausz, G. Gervasi, G. Forti and E. Baldi. 1995. Stimulation of protein tyrosine phosphorylation by platelet-activating factor and progesterone in human spermatozoa. Mol. Cell. Endocrinol., 108(1-2): 35-42. DOI: 10.1016/0303-7207(95)92576-a
Mahony, M.C. and T.Y. Gwathmey. 1999. Protein tyrosine phosphorylation during hyperactivated motility of cynomolgus monkey (Macaca fascicularis) spermatozoa. Biol. Reprod., 60(5): 1239-1243. DOI: 10.1095/biolreprod60.5.1239
Meizel, S. and K.O. Turner. 1993. Initiation of the human sperm acrosome reaction by thapsigargin. J. Exp. Zool., 267(3): 350-355. DOI: 10.1002/jez.1402670312
Menzel, V.A., E. Hinsch, W. Hagele and K.D. Hinsch. 2007. Effect of genistein on acrosome reaction and zona pellucida binding independent of protein tyrosine kinase inhibition in bull. Asian J. Androl., 9(5): 650-658. DOI: 10.1111/j.1745-7262.2007.00240.x
Nakashima, S., T. Koike and Y. Nozawa. 1991. Genistein, a protein tyrosine kinase inhibitor, inhibits thromboxane A2-mediated human platelet responses. Mol. Pharmacol., 39(4): 475-480. DOI: 10.1016/s0026-895x(25)11014-6
Otter, T., S.M. King and G.B. Witman. 1987. A two-step procedure for efficient electrotransfer of both high-molecular-weight (greater than 400,000) and low-molecular-weight (less than 20,000) proteins. Anal. Biochem., 162(2): 370-377. DOI: 10.1016/0003-2697(87)90406-4
Parrish, J.J., J. Susko-Parish, M.A. Winer and N.L. First. 1988. Capacitation of bovine sperm by heparin. Biol. Reprod., 38(5): 1171-1180. DOI: 10.1095/biolreprod38.5.1171
Pukazhenthi, B.S., D.E. Wildt, M.A. Ottinger and J. Howard. 1998. Inhibition of domestic cat spermatozoa acrosome reaction and zona pellucida penetration by tyrosine kinase inhibitors. Mol. Reprod. Dev., 49(1): 48-57. DOI: 10.1002/(SICI)1098-2795(199801)49:1<48::AID-MRD6>3.0.CO;2-O
Queiroz, E.K. and W. Waissmann. 2006. Occupational exposure and effects on the male reproductive system. Cad. Saúde Pública, 22(3): 485-493. DOI: 10.1590/s0102-311x2006000300003
Revelli, A., G. Soldati, C. Costamagna, O. Pellerey, E. Aldieri, M. Massobrio, A. Bosia and D. Ghigo. 1999. Follicular fluid proteins stimulate nitric oxide (NO) synthesis in human sperm: A possible role for NO in acrosomal reaction. J. Cell. Physiol., 178(1): 85-92. DOI: 10.1002/(SICI)1097-4652(199901)178:1<85::AID-JCP11>3.0.CO;2-Y
Roberts, K.P., J.A. Wamstad, K.M. Ensrud and D.W. Hamilton. 2003. Inhibition of capacitation-associated tyrosine phosphorylation signaling in rat sperm by epididymal protein Crisp-1. Biol. Reprod., 69(2): 572-581. DOI: 10.1095/biolreprod.102.013771
Rodriguez, P.C. and M.T. Beconi. 2009. Peroxynitrite participates in mechanisms involved in capacitation of cryopreserved cattle. Anim. Reprod. Sci., 110(1-2): 96-107. DOI: 10.1016/j.anireprosci.2007.12.017
Rodriguez, P.C., C.M. O’Flaherty, M.T. Beconi and N.B. Beorlegui. 2005. Nitric oxide induces acrosome reaction in cryopreserved bovine spermatozoa. Andrologia, 37(5): 166-172. DOI: 10.1111/j.1439-0272.2005.00674.x
Ronis, M.J. 2016. Effects of soy containing diet and isoflavones on cytochrome P450 enzyme expression and activity. Drug Metab. Rev., 48(3): 331-341. DOI: 10.1080/03602532.2016.1206562
Roy, S.C. and S.K. Atreja. 2008. Effect of reactive oxygen species on capacitation and associated protein tyrosine phosphorylation in buffalo (Bubalus bubalis) spermatozoa. Anim. Reprod. Sci., 107(1-2): 68-84. DOI: 10.1016/j.anireprosci.2007.06.024
Sengoku, K., K. Tamate, T. Yoshida, Y. Takaoka, T. Miyamoto and M. Ishikawa. 1998. Effects of low concentrations of nitric oxide on the zona pellucida binding ability of human spermatozoa. Fertil. Steril., 69(3): 522-527. DOI: 10.1016/s0015-0282(97)00537-2
Siddique, R.A. and S.K. Atreja. 2012. Effect of Spermine-NONOate on acrosome reaction and associated protein tyrosine phosphorylation in Murrah buffalo (Bubalus bubalis) spermatozoa. Anim. Reprod. Sci., 131(1-2): 81-87. DOI: 10.1016/j.anireprosci.2012.02.010
Siddique, R.A. and S.K. Atreja. 2013. Effect of L-Arginine and spermine-NONOate on motility, viability, membrane integrity and lipid peroxidation of Murrah buffalo (Bubalus bubalis) spermatozoa. Livest. Sci., 153(1-3): 147-153. DOI: 10.1016/j.livsci.2013.01.007
Siddique, R.A., S. Atreja, N. Ali and K.P. Singh. 2021. Physiological Concentration of spermine-NONOate induces acrosome reaction in Bubalus bubalis spermatozoa. International Journal of Livestock Research, 11(2): 165-174. DOI: 10.5455/ijlr.20200924065003
Siddique, R.A., Shabana, N. Ali, M.K. Bharti, A. Kumar, A. Kumar and T. Ambwani. 2019. Nitric oxide: A prime signaling molecule in bovine male reproduction. International Journal of Livestock Research, 9(8): 49-74. DOI: 10.5455/ijlr.20180320063007
Suraj, K. and S.K. Atreja. 2000. Heparin induced capacitation of buffalo spermatozoa. In Compendium of 69th Annual Meeting of the Society of Biological Chemists (India) at Science City, Calcutta, India. 201p.
Tesarik, J., A. Carreras and C. Mendoza. 1996. Single cell analysis of tyrosine kinase dependent and independent Ca2+ fluxes in progesterone induced acrosome reaction. Mol. Hum. Reprod., 2(4): 225-232. DOI: 10.1093/molehr/2.4.225
Tuli, H.S., M.J. Tuorkey, F. Thakral, K. Sak, M. Kumar, A.K. Sharma, U. Sharma, A. Jain, V. Aggarwal and A. Bishayee. 2019. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol., 10: 1336. DOI: 10.3389/fphar.2019.01336
Visconti, P.E., H. Galantino-Homer, G.D. Moore, J.L. Bailey, X. Ning and M. Fornes. 1998. The molecular basis of sperm capacitation. J. Androl., 19(2): 242-248. https://onlinelibrary.wiley.com/doi/pdf/10.1002/j.1939-4640.1998.tb01994.x
Yoshida, K., Y. Mizukami and M. Kitakaze. 1999. Nitric oxide mediates protein kinase C isoform translocation in rat heart during postischemic reperfusion. Biochim. Biophys. Acta, 1453(2): 230-238. DOI: 10.1016/s0925-4439(98)00105-7








.png)








