Prevalence and antibiogram of Listeria monocytogenes in retailed buffalo raw meat and mince with a protection trial using nisin, and gingerol
Keywords:
Bubalus bubalis, buffaloes, buffalo meat, Listeria monocytogenes, multidrug resistance, nisin, gingerolAbstract
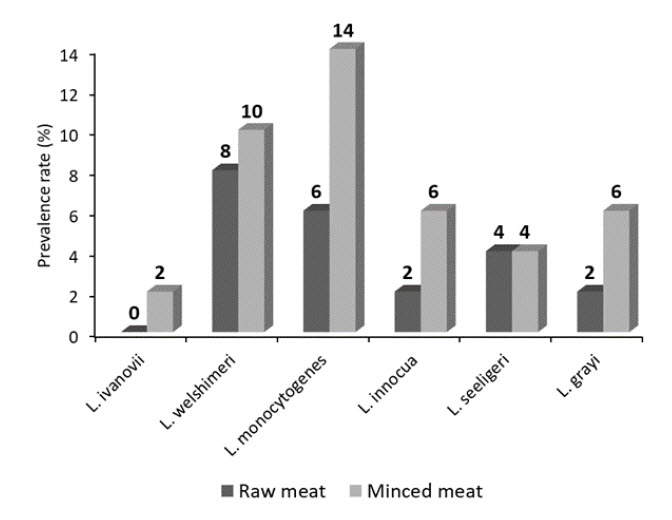
Buffalo meat is an emerging source of high-quality animal protein. However, the role of the buffalo meat in the transmission of foodborne pathogens such as Listeria monocytogenes (L. monocytogenes) is scarcely investigated. Therefore, this study aimed at investigation of the prevalence rates of Listeria spp., particularly L. monocytogenes in retailed buffalo meat and buffalo mince in the Egyptian markets. In addition, antimicrobial susceptibility testing of the recovered L. monocytogenes isolates was further screened. Furthermore, antilisterial effects of two natural food additives, namely nisin, and gingerol were further examined. The obtained results of the present study revealed an overall isolation rates of Listeria spp. and L. monocytogenes from all examined samples at 34%, and 10%, respectively. Serological identification of the isolated Listeria spp. revealed recovery of six Listeria spp. namely, L. ivanovii, L. welshimeri, L. innocua, L. seeligeri, L. grayi, and L. monocytogenes. L. monocytogenes was isolated at 6%, and 14% from the examined buffalo meat and buffalo mince, respectively. The recovered L. monocytogenes had multidrug resistance profiling. Nisin and gingerol had clear antilisterial activities. As nisin achieved reduction rates of 11.36%, and 44.84% at 1%, and 2%, respectively; while gingerol achieved reduction rates of 8.44%, and 36.17% at 1%, and 2%, respectively. Therefore, it is recommended to use such food additives for the control of L. monocytogenes in the meat industry.
Downloads
Metrics
References
Ahmed, H.A., M.A. Hussein and A.M. El-Ashram. 2013. Seafood a potential source of some zoonotic bacteria in Zagazig, Egypt, with the molecular detection of Listeria monocytogenes virulence genes. Vet. Ital., 49(3): 299-308. DOI: 10.12834/VetIt.1305.05
American Public Health Association, APHA. 2001. Compendium of Methods for the Microbiological Examination of Food, 4th ed. American Public Health Association, Washington, USA.
Avery, S.M. and S. Buncic. 1997. Antilisterial Effects of a sorbate-nisin combination in vitro and on packaged beef at refrigeration temperature. J. Food Protech., 60(9): 1075-1080. DOI: 10.4315/0362-028X-60.9.1075
Barbuddhe, S.B., S.P. Chaudhari and S.V. Malik. 2002. The occurrence of pathogenic Listeria monocytogenes and antibodies against listeriolysin-O in buffaloes. J. Vet. Med. B., 49(4): 181-184. DOI: 10.1046/j.1439-0450.2002.00527.x
Castellazzi, M.L., P. Marchisio and S. Bosis. 2018. Listeria monocytogenes meningitis in immunocompetent and healthy children: a case report and a review of the literature. Ital. J. Pediatr., 44(1): 152.
Clinical and Laboratory Standards Institute. 2021. Performance Standards for Antimicrobial Susceptibility Testing, 31st ed. Clinical and Laboratory Standards Institute, Villanova PA, USA.
Cockrill, W.R. 1981. The water buffalo: A review. Brit. Vet. J., 137(1): 8-16. DOI: 10.1016/s0007-1935(17)31782-7.
Darwish, W.S., E.A. Eldaly, M.T. El-Abbasy. 2013. Antibiotic residues in food: The African scenario. Jpn. J. Vet. Res., 61: S13-S22.
Davies, E.A., H.E. Bevis and J. Delves-Broughton. 1997. The use of the bacteriocin, nisin, as a preservative in ricotta-type cheeses to control the food-borne pathogen Listeria monocytogenes. Lett. Appl. Microbiol., 24(5): 343-346. DOI: 10.1046/j.1472-765x.1997.00145.x
De Cesare, A., A. Parisi, R. Mioni, D. Comin, A. Lucchi and G. Manfreda. 2017. Listeria monocytogenes circulating in rabbit meat products and slaughterhouses in Italy: Prevalence data and comparison among typing results. Foodborne Pathog. Dis., 14(3): 167-176. DOI: 10.1089/fpd.2016.2211
Dimic, G.R., S.D. Tanackov, O.O. Jovanov, D.D. Cvetkovic and S.L. Velicanski. 2010. Presence of Listeria species in fresh meats from retail markets in Serbia. Acta Periodica Technologica, 41(41): 1-6. DOI: 10.2298/APT1041001D
FAO/WHO. 2010. Risk Assessment of Listeria monocytogenes in Ready-to-Eat Food, 2004. Food and Agriculture Organization. Available on: http://www.fao.org/docrep/010/y5394e/y5394e00.htm.
Govaris, A., N. Solomakos, A. Pexara and P.S. Chatzopoulou. 2010. The antimicrobial effect of oregano essential oil, nisin and their combination against Salmonella Enteritidis in minced sheep meat during refrigerated storage. Int. J. Food Microbiol., 137(2-3): 175-80. DOI: 10.1016/j.ijfoodmicro.2009.12.017
Hassan, Z., E. Purwati, S. Radu, R.A. Rahim and G. Rusul. 2001. Prevalence of Listeria spp. and Listeria monocytogenes in meat and fermented fish in Malaysia. SE. Asian J. Trop. Med., 32(2): 402-407.
He, L., L. Zou, Q. Yang, J. Xia, K. Zhou, Y. Zhu, X. Han, B. Pu, B. Hu, W. Deng and S. Liu. 2016. Antimicrobial activities of Nisin, tea polyphenols, and chitosan and their combinations in chilled mutton. J. Food Sci., 81(6): M1466-M1471. DOI: 10.1111/1750-3841.13312
Khan, I., J. Khan, S. Miskeen, C.N. Tango, Y.S. Park and D.H. Oh. 2016. Prevalence and control of Listeria monocytogenes in the food industry - A review. Czech J. Food Sci., 34(6): 469-487. DOI: 10.17221/21/2016-CJFS
Liu, Y., W. Sun, T. Sun, L.G.M. Gorris, X. Wang, B. Liu and Q. Dong. 2020. The prevalence of Listeria monocytogenes in meat products in China: A systematic literature review and novel meta-analysis approach. Int. J. Food Microbiol., 312: 108358. DOI: 10.1016/j.ijfoodmicro.2019.108358
Maćkiw, E., D. Korsak, J. Kowalska, B. Felix, M. Stasiak, K. Kucharek and J. Postupolski. 2021. Incidence and genetic variability of Listeria monocytogenes isolated from vegetables in Poland. Int. J. Food Microbiol., 339: 109023. DOI: 10.1016/j.ijfoodmicro.2020.109023
Matle, I., K.R. Mbatha and E. Madoroba. 2020. A review of Listeria monocytogenes from meat and meat products: Epidemiology, virulence factors, antimicrobial resistance and diagnosis. Onderstepoort J. Vet., 87(1): e1-e20. DOI: 10.4102/ojvr.v87i1.1869
Mehdizadeh, T., H. Tajik, A.M. Langroodi, R. Molaei and A. Mahmoudian. 2020. Chitosan-starch film containing pomegranate peel extract and Thymus kotschyanus essential oil can prolong the shelf life of beef. Meat Sci., 163: 108073. DOI: 10.1016/j.meatsci.2020.108073
Mohamed, Y., W.W. Reda, K. Abdel-Moein, K.A. Abd El-Razik, A.M.A. Barakat, H.A. El Fadaly, N.A. Hassanain and A.G. Hegazi. 2016. Prevalence and phylogenetic characterization of Listeria monocytogenes isolated from processed meat marketed in Egypt. Journal of Genetic Engineering and Biotechnology, 14(1): 119-123. DOI: 10.1016/j.jgeb.2016.06.001
Park, M., J. Bae and D.S. Lee. 2008. Antibacterial activity of [10]-gingerol and [12]-gingerol isolated from ginger rhizome against periodontal bacteria. Phytother. Res., 22(11): 1446-1449. DOI: 10.1002/ptr.2473
Pearson, A.M. and F.W. Tauber. 1984. Analytical methods. p. 351-388. In Processed Meats. he AVI Publishing Company, Inc., Springer, Dordrecht, Netherland.
Preiato, D. 2020. All You Need to Know About Bison Meat. Available on: https://www.healthline.com/nutrition/bison-meat-nutrition.
Rahimi, E., F. Yazdi and H. Farzinezhadizadeh. 2012. Prevalence and antimicrobial resistance of Listeria species isolated from different types of raw meat in Iran. J. Food Protect., 75(12): 2223-2227. DOI: 10.4315/0362-028X.JFP-11-565
Shahbazi, Y., N. Karami and N. Shavisi. 2018. Effect of Ziziphora clinopodioides essential oil on shelf life and fate of Listeria monocytogenes and Staphylococcus aureus in refrigerated chicken meatballs. J. Food Safety, 38: 1-10. DOI: 10.1111/JFS.12394
Shamloo, E., H. Hosseini, Z.A. Moghadam, M.H. Larsen, A. Haslberger and M. Alebouyeh. 2019. Importance of Listeria monocytogenes in food safety: a review of its prevalence, detection, and antibiotic resistance. Iran J. Vet. Res., 20(4): 241-254.
Singh, A., Yadav, S., S. Singh and P. Bharti. 2010. Prevalence of Salmonella in chicken eggs collected from poultry farms and marketing channels and their antimicrobial resistance. Food Res. Inter., 43(8): 2027-2030.
Tang, H., W.S. Darwish, W.R. El-Ghareeb, N.A. Al-Humam, L. Chen, R.M. Zhong, Z.J. Xiao and J.K. Ma. 2020. Microbial quality and formation of biogenic amines in the meat and edible offal of Camelus dromedaries with a protection trial using gingerol and nisin. Food Science and Nutrition, 8(4): 2094-2101. DOI: 10.1002/fsn3.1503
Vongkamjan, K., S. Benjakul, H.T. Kim Vu and V. Vuddhakul. 2017. Longitudinal monitoring of Listeria monocytogenes and Listeria phages in seafood processing environments in Thailand. Food Microbiol., 66: 11-19. DOI: 10.1016/j.fm.2017.03.014
Weller, D., A. Andrus, M. Wiedmann. 2015. Listeria booriae sp. nov. and Listeria newyorkensis sp. nov., from food processing environments in the USA. Int. J. Syst. Evol. Micr., 65(1): 286-292. DOI: 10.1099/ijs.0.070839-0
Yucel, N., S. Citak and M. Onder. 2005. Prevalence and antibiotic resistance of Listeria species in meat products in Ankara, Turkey. Food Microbiol., 22(2-3): 241-245. DOI: 10.1016/j.fm.2004.03.007
Zhang, Y., E. Yeh, G. Hall, J. Cripe, A.A. Bhagwat and J. Meng. 2007. Characterization of Listeria monocytogenes isolated from retail foods. Inter. J. Food Microbiol., 113: 47-53. DOI: 10.1016/j.ijfoodmicro.2006.07.010




.png)








