Comparison of ultrasonographic measures of reproductive tract in Thai swamp buffalo heifers and cows
DOI:
https://doi.org/10.56825/bufbu.2023.4245554Keywords:
Bubalus bubalis, buffaloes, Thai swamp buffalo, reproductive tract, ultrasonography, ovarian componentAbstract
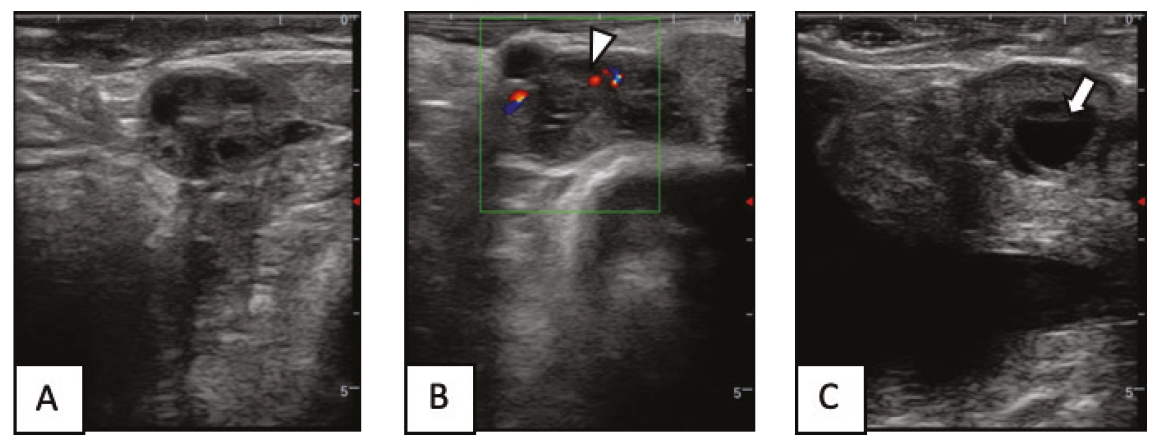
Although artificial insemination (AI) technology is widely used in buffalo breeding in Thailand, AI in buffaloes has a lower conception rate compared to AI in beef cattle. It is crucial to understand the development of the ovary and reproductive system of buffalo heifers and cows. The objective of the present study was to evaluate the reproductive tract characteristic using ultrasound measurements during the luteal and follicular phases in Thai swamp buffalo heifers and cows. The studies of reproductive tract were conducted in buffalo cows (n=8; BCS=3.38) and heifers (n=8; BCS=3.25). The reproductive tract was evaluated by measuring the size of the ovary, dominant follicle, CL, uterine horn, cervix, and vulva. The buffalo cows had larger diameter of the cervix and vulvar width (P<0.05) compared with those of heifers. In the follicular phase, buffalo cows had a mean ovarian diameter (ipsilateral POF) of 2.35 cm, and heifers had a mean ovarian diameter of 2.24 cm (P>0.05). The average preovulatory follicle diameter was 1.29 cm in buffalo cows and 1.18 cm in heifers (P>0.05). In the luteal phase, the mean ovarian diameter (ipsilateral CL) of buffalo cows was significantly larger than heifers (P<0.05; 2.46 vs. 2.09 cm, respectively). The diameters of the CL in buffalo heifers and cows were 1.27 and 1.47 cm, respectively (P>0.05). Buffalo cows and heifers have no differences in ovarian components in the follicular and luteal phases, but there are differences in the size of the reproductive tract, which reproductive tract of buffalo cows is larger than heifers.
Downloads
Metrics
References
Alapati, A., S.R. Kapa, S. Jeepalyam, S.M. Rangappa and K.R. Yemireddy. 2010. Development of the body condition score system in Murrah buffaloes: validation through ultrasonic assessment of body fat reserves. J. Vet. Sci., 11(1): 1-8. DOI: 10.4142/jvs.2010.11.1.1
Bó, G., L. Cutaia, L. Peres, D. Pincinato, D. Maraña and P. Baruselli. 2007. Technologies for fixed-time artificial insemination and their influence on reproductive performance of Bos indicus cattle. Society for Reproduction and Fertility, 64: 223-236. DOI: 10.5661/rdr-vi-223
Baruselli, P.S., R.G. Mucciolo, J.A. Visintin, W.G. Viana, R.P. Arruda, E.H. Madureira, C.A. Oliveira and J.R. Molero-Filho. 1997. Ovarian follicular dynamics during the estrous cycle in buffalo (Bubalus bubalis). Theriogenology, 47(8): 1531-1547. DOI: 10.1016/s0093-691x(97)00159-3
Chaikhun, T., T. Tharasanit, J. Rattanatep, F. De Rensis and M. Techakumphu. 2010. Fertility of swamp buffalo following the synchronization of ovulation by the sequential administration of GnRH and PGF2 alpha combined with fixed-timed artificial insemination. Theriogenology, 74(8): 1371-1376. DOI: 10.1016/j.theriogenology.2010.06.007
Dahlen, C., J. Larson and G.C. Lamb. 2014. Impacts of reproductive technologies on beef production in the United States. Adv. Exp. Med. Biol., 742: 97-114. DOI: 10.1007/978-1-4614-8887-3_5
Drost, M. 2007. Bubaline versus bovine reproduction. Theriogenology, 68(3): 447-449. DOI: 10.1016/j.theriogenology.2007.04.012
Fontes, P.L.P. and N. Oosthuizen. 2022. Applied use of doppler ultrasonography in bovine reproduction. Frontiers in Animal Science, 3. DOI: 10.3389/fanim.2022.912854
Gearhart, M.A., C.R. Curtis, H.N. Erb, R.D. Smith, C.J. Sniffen, L.E. Chase and M.D. Coope. 1990. Relationship of changes in condition score to cow health in Holsteins. J. Dairy Sci., 73(11): 3132-3140. DOI: 10.3168/jds.S0022-0302(90)79002-9
Kähn, W. 1992. Ultrasonography as a diagnostic tool in female animal reproduction, Anim. Reprod. Sci., 28(1-4): 1-10. DOI: 10.1016/0378-4320(92)90085-R
Lamb, G., V. Mercadante, D. Henry, P. Fontes, C. Dahlen, J. Larson and N. Dilorenzo. 2016. Invited review: advantages of current and future reproductive technologies for beef cattle production. Professional Animal Scientist, 32(2): 162-171. DOI: 10.15232/pas.2015-01455
Mesquita, N.F., R. Maculan, L.F. Maciel, N. Alves, R.R. De Carvalho, G.M. Moreira and J.C. De Souza. 2016. Vulvar width and rima length as predictors of the ovarian follicular reserve in bovine females. J. Reprod. Develop., 62(6): 587-590. DOI: 10.1262/jrd.2016-059
Mondal, S., B.S. Prakash and P. Palta. 2007. Endocrine aspects of oestrous cycle in buffaloes (Bubalus bubalis): An overview. Asian Austral. J. Anim., 20(1): 124-131. DOI: 10.5713/ajas.2007.124
Nguyen, B.X., N.T. Uoc, N.T. Thanh, N.V. Linh, L.C. Bui, N.V. Hanh and D.D. Long. 2014. Reproduction in the Swamp Buffalo (Bubalus bubalis), p. 604-602. In Purohit, G.N. (edn.) Bubaline Theriogenology, International Veterinary Information Service, New York, USA.
Singh, J., A.S. Nanda and G.P. Adams. 2000. The reproductive pattern and efficiency of female buffaloes. Anim. Reprod. Sci., 60-61: 593-604. DOI: 10.1016/s0378-4320(00)00109-3
Thakur, R., K. Niranjan, K. Pankaj, C. Shailendra and P. Navin. 2013. Heat detection techniques in cattle and buffalo. Vet. World, 6(7): 363-369. DOI: 10.5455/vetworld.2013.363-369
Van Engelen, E., M.A.M. Taverne, M.E. Everts, G.C. Van der Weijden, A. Doornenbal and V.B. Dwarkasing. 2007. Cervical diameter in relation to uterine and cervical EMG activity in early postpartum dairy cows with retained placentas after PGF2alpha induced calving. Theriogenology, 68(2): 213-222. DOI: 10.1016/j.theriogenology.2007.04.054
Yindee, M., M. Techakumphu, C. Lohachit, S. Sirivaidyapong, A. Na-Chiangmai, H. Rodriguez-Martinez, G. Van der Weyden and B. Colenbrander. 2011. Follicular dynamics and oestrous detection in Thai postpartum swamp buffaloes (Bubalus bubalis). Reprod. Domest. Anim., 46(1): e91-e96. DOI: 10.1111/j.1439-0531.2010.01647.x








.png)








