Characterization of coding areas of SPGA11B gene in Murrah bulls
DOI:
https://doi.org/10.56825/bufbu.2022.4122814Keywords:
Bubalus bubalis, buffaloes, SPAG11 gene, characterization, DNA sequencing Murrah bullsAbstract
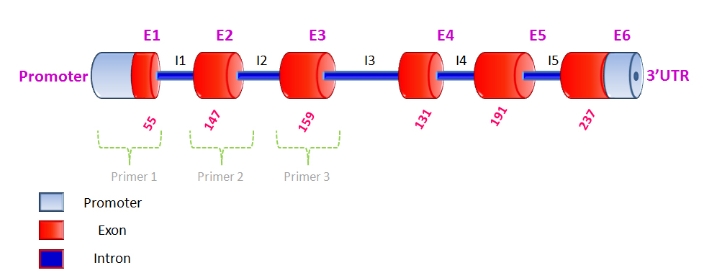
The SPAG11 gene is one of pivotal molecules in reproduction as it takes part in spermatozoa maturation, acquiring motility, capacitation, and egg-sperm interaction as well. The current study was aimed to characterize SPAG11 gene in Indian Murrah bulls through direct DNA sequencing approach. Genomic DNA from Murrah animals were isolated from 130 Murrah bulls and amplified using three sets of forward and reverse primers which were based on reference sequence (Genbank accession no. AC_000164.1) of Bos taurus covering entire coding region of SPAG11B gene. The PCR products of 563, 340 and 373, bp covering exons 1 to 3 were subjected to sequencing and subsequently ClustalW analysis revealed the substitution at 34 positions and a single stretch of 22 bp deletion in comparison to the Bos taurus reference sequence. Total seven novel SNPs were observed as two in the coding region and five in 5ˈUTR. However, only one of SNPs resulted in amino acid substitution viz. p.1279 arginine to tryptophan in translated protein in Murrah buffaloes. Sequence alignment and homology across species for the targeted nucleotide sequence of SPAG11 gene in Murrah bulls was done by nucleotide BLAST (NCBI) that showed maximum identity of 97% with mRNA of Bos taurus and Capra hircus followed by 96% homology with Bos indicus and Bison bison and 95% homology with Ovis aries and Bos mutus.
Downloads
Metrics
References
Bagnicka, E., N. Strzałkowska, K. Flisikowski, T. Szreder, A. Jorwik, B. Prusak, J. Krzyzewski and L. Zwierzchowski. 2007. The polymorphism in the β4 defensin gene and its association with production and somatic cell count in Holstein-Friesian cows. J. Anim. Breed. Genet., 124(3): 150-156. DOI: 10.1111/j.1439-0388.2007.00649.x
Bateman, A., R.J. MacLeod, P. Lembessis, J. Hu, F. Esch and S. Solomon. 1996. The isolation and characterization of a novel corticostatin/ defensin-like peptide from the kidney. J. Biol. Chem., 271(18): 10654-10659. DOI: 10.1074/jbc.271.18.10654
Bedford, J.M. 1967. Fertile life of rabbit spermatozoa in rat uterus. Nature, 213: 1097-1099. DOI: 10.1038/2131097a0
Breitbart, H. 2002. Intracellular calcium regulation in sperm capacitation and acrosomal reaction. Mol. Cell. Endocrinol., 187(1): 139-144. DOI: 10.1016/s0303-7207(01)00704-3
Green, M.R. and J. Sambrook. 2012. Molecular Cloning: A laboratory Manual, 4th ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, USA.
Guraya, S.S. 2000. Cellular and molecular biology of capacitation and acrosome reaction in spermatozoa. Int. Rev. Cytol., 199: 1-64. DOI: 10.1016/S0074-7696(00)99001-6
Hamil, K.G., P. Sivashanmugam, R.T. Richardson, G. Grossman, S.M. Ruben, J.L. Mohler, P. Petrusz, M.G. Orand, F.S. French and S.H. Hall. 2000. HE2β and HE2γ, new members of an epididymis-specific family of androgen-regulated proteins in the human. Endocrinology, 141(3): 1245-1253. DOI: 10.1210/endo.141.3.7389
Harighi, M.F., H. Wahid, P.C. Thomson, M.Y. Rafii and F.F.A. Jesse. 2019. Novel SNPs in the SPAG11 gene and association with testicular biometric variables in Boer goats and application of the levelled-container technique. Anim. Reprod. Sci., 208: 106-113. DOI: 10.1016/j.anireprosci.2019.106113
Heckmann, J.M., H. Uwimpuhwe, R. Ballo, M. Kaur, V.B. Bajic and S. Prince. 2010. A functional SNP in the regulatory region of the decay-accelerating factor gene associates with extraocular muscle pareses in myasthenia gravis. Genes Immun., 11: 1-10. DOI: 110.1038/gene.2009.61
Hoskins, D.D., T.S. Acott, L. Critchlow and S. Vijayaraghavan. 1983. Studies on the roles of cyclic AMP and calcium in the development of bovine sperm motility. J. Submicrosc. Cytol., 15(1): 21-27.
Jayendran, R.S., H.H. Vandervent, Perez Peleae, Z.M., B.G. Crabo and L.J.D. Zanevald. 1984. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to their semen characteristics. J. Reprod. Fertil., 70(1): 219-225. DOI: 10.1530/jrf.0.0700219
Jeulin, C. and M.L. Lewin. 1996. Role of free L-carnitine and acetyl-L-carnitine in post-gonadal maturation of mammalian spermatozoa. Hum. Reprod., 2(2): 87-102. DOI: 10.1093/humupd/2.2.87
Li, P., H.C. Chan, B. He, S.C. So, Y.W. Chung, Q. Shang, Y.D. Zhang and Y.L. Zhang. 2001. An antimicrobial peptide gene found in the male reproductive system of rats. Science, 291(5509): 1783-1785. DOI: 10.1126/science.1056545
MacLeod, R.J., J.R. Hamilton, A. Bateman, D. Belcourt, J. Hu, H.P. Bennett and S. Solomon. 1991. Corticostatic peptides cause nifedipine-sensitive volume reduction in jejunal villus enterocytes. P. Natl. Acad. Sci. USA., 88(2): 552-556. DOI: 10.1073/pnas.88.2.552
Orgebin-Crist, M.C. 1967. Sperm maturation in rabbit epididymis. Nature, 216: 816-818. DOI: 10.1038/216816a0
Vijayaraghavan, S. and D.D. Hoskins. 1988. Low molecular weight factor in bovine caudal epididymal fluid that stimulates calcium uptake in caput spermatozoa. Gamete Res., 20(3): 343-352. DOI: 10.1002/mrd.1120200309
Xu, W., X. Zhang, W. Chen, K.L. Fok, D.K. Rowlands, Y.L. Chui and H.C. Chan. 2010. Immunization with Bin1b decreases sperm motility with compromised fertility in rats. Fertil. Steril., 93(3): 952-958. DOI: 10.1016/j.fertnstert.2008.10.066
Yenugu, S., K.G. Hamil, F.S. French and S.H. Hall. 2006. Antimicrobial actions of human and macaque sperm associated antigen (SPAG) 11 isoforms: influence of the N-terminal peptide. Mol. Cell. Biochem., 284(1-2): 25-37. DOI: 10.1007/s11010-005-9009-2
Zhou, C.X., Y.L. Zhang, L. Xiao, M. Zheng, K.M. Leung, M.Y. Chan, P.S. Lo, L.L. Tsang, H.Y. Wong, L.S. Ho, Y.W. Chung and H.C. Chan. 2004. An epididymis-specific beta-defensin is important for the initiation of sperm maturation. Nat. Cell Biol., 6(5): 458-464. DOI: 10.1038/ncb1127








.png)








