Single nucleotide polymorphism, haplotypes and genotypes of Mannose-binding lectin (MBL1) gene and their association with clinical mastitis in Murrah buffalo
DOI:
https://doi.org/10.56825/bufbu.2023.4225055Keywords:
Bubalus bubalis, buffaloes, immumne, nucleotide, polymorphism, haplotypesAbstract
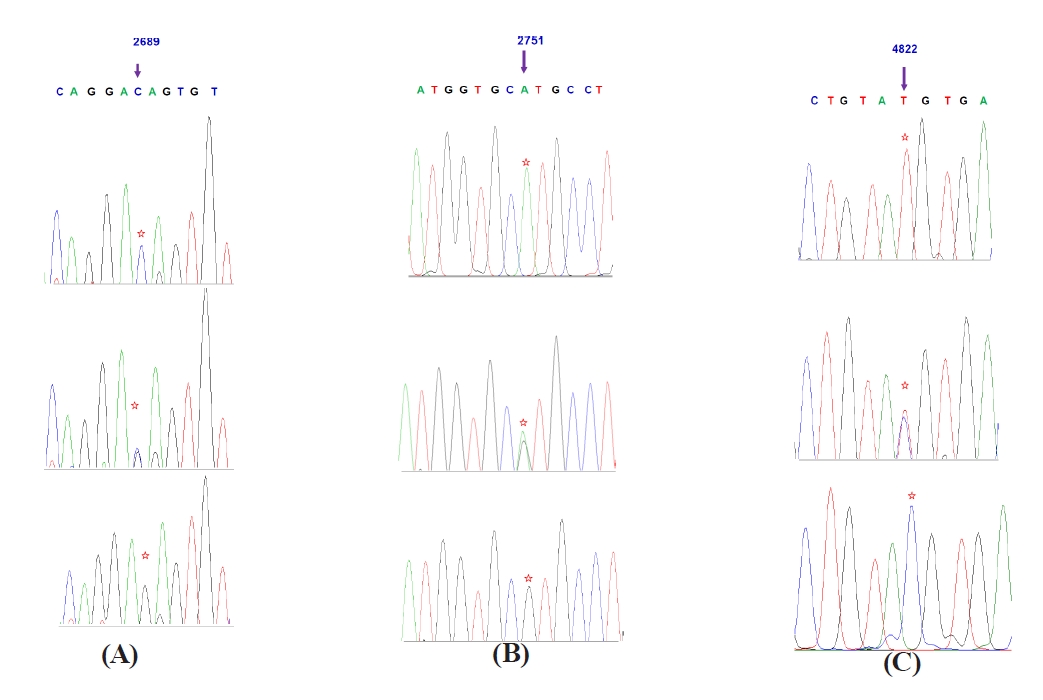
The Mannose-binding lectin (MBL1) gene is an important component of immune response system. It plays vital role in activation of the complement system and act as chief defense molecule of host cell to provide protection against various diseases. In the recent study, six novel single nucleotide polymorphisms (SNPs), at 2689G>C, 2751A>G, 4822T>C, 4853A>G, 4855T>C, and 4978T>C resulted in 2 non synonymous type of change at 4853A>G, 4855T>C resulting in one amino acid substitution Ser150Gly of MBL1 protein and their association with clinical mastitis were investigated in two hundred (200) Murrah buffaloes. These SNPs in MBL1 were genotyped by using the polymerase chain reaction (PCR) and Deoxyribose nucleic acid (DNA) sequencing methods in order to reveal the association with clinical mastitis. SHEsis software tool was used for construction of haplotypes and Linkage disequilibrium analysis. Twenty one (21) haplotypes were constructed. Allelic association analysis showed that C allele of 2689 G>C, A allele of 2751 A>G, C allele of 4822 T>C, A allele of 4853 A>G, C allele of 4855 T>C, and C allele of 4978 T>C had significant association with increased risk of clinical mastitis in Murrah buffaloes (P<0.05). Murrah buffaloes with CG genotype of 2689 G>C, AG or GG genotype of 2751 A>G, CC genotype of 4822T>C, AG or GG genotype of 4853 A>G, TC or CC genotype of 4855 T>C and CC genotype of 4978 T>C loci had significantly lower incidence of clinical mastitis compared to their counter genotypes. Haplotype association analysis showed that Hap21 (TATTGA) was found significantly related to higher risk of clinical mastitis (P<0.05). Hap14 (TGTTGG) and Hap5 (CGCCCA) were found significantly associated with lower risk of clinical mastitis in Murrah buffalo (P<0.05).
Downloads
Metrics
References
Asaf, V.N.M., B. Bhushan, M. Panigrahi, P. Dewangan, A. Kumar, P. Kumar and G.K. Gaur. 2014a. Association study of genetic variants at single nucleotide polymorphism rs109231409 of mannose-binding lectins 1 gene with mastitis susceptibility in Vrindavani crossbred cattle, Vet. World, 7(10): 807-810. Available on: http://www.veterinaryworld.org/Vol.7/October-2014/10.pdf
Bouwman, L.H., B.O. Roep and A. Roos. 2006. Mannose-binding lectin: Clinical implications for infection, transplantation, and autoimmunity. Hum. Immunol., 67(4-5): 247-256. DOI: 10.1016/j.humimm.2006.02.030
Eisen, D.P. and R.M. Minchinton. 2003. Impact of mannose-binding lectin on susceptibility to infectious diseases. Clin. Infect. Dis., 37(11): 1496-1505. DOI: 10.1086/379324
Gjerstorff, M., S. Hansen, B. Jensen, B. Dueholm, P. Horn, C. Bendixenand and U. Holmskov. 2004. The genes encoding bovine SP-A, SP-D, MBL-A, conglutinin, CL-43 and CL-46 form a distinct collectin locus on Bos taurus chromosome 28 (BTA28) at position q.1.8-1.9. Anim. Genet., 35(4): 333-337. DOI: 10.1111/j.1365-2052.2004.01167.x
Hall, T.A. 1999. Bioedit: A user- friendly biological sequence alignment editor and anlaysis program for windows 95/98/NT. Nucl. Acid. S., 41: 95-98.
Janeway C.A., P. Travers, M. Walport and M. Shlomchik. 2005. Immunobiology. Garland Publishing, New York, USA.
Lillie, B.N., J.D. Hammermueller, J.I. MacInnes, M. Jacques and M.A. Hayes. 2006. Porcine mannan-binding lectin A binds to Actinobacillus suis and Haemophilus parasuis. Dev. Comp. Immunol., 30(10): 954-965. DOI: 10.1016/j.dci.2005.12.008
Liu, J., Z. Ju, Q. Li, J. Huang, R. Li, J. Li, L. Ma, J. Zhong and C. Wang. 2011. Mannose-binding lectin 1 haplotypes influence serum MBL-A concentration, complement activity, and milk production traits in Chinese Holstein cattle. Immunogenetics, 63(11): 727-742. DOI: 10.1007/s00251-011-0548-2
Neth, O., D.L. Jack, A.W. Dodds, H. Holzel, N.J. Klein and M.W. Turner. 2000. Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun., 68(2): 688-693. DOI: 10.1128/IAI.68.2.688-693.2000
Ruegg, P.L. 2003. Investigation of mastitis problems on farms. Vet. Clin. N. Am. Food A., 19(1): 47-73. DOI: 10.1016/s0749-0720(02)00078-6
Sambrook, J. and D.W. Russell. 1989. Preparation and analysis of eukaryotic DNA. In Molecular Cloning: A Laboratory Manual, 3rd ed. Cold Spring Harbor Laboratory Press, New York, USA.
Shergojry, S.A., A. Verma, I.D. Gupta and N.A. Mir. 2021. Association analysis of MBL1 gene SNPs, genotype and haplotypes with clinical mastitis in Murrah buffaloes. Journal of Experimental Agriculture International, 43(6), 35-44. DOI: 10.9734/jeai/2021/v43i630700
Shergojry, S.A., A. Verma, M. Ghani, I.D. Gupta and N.A. Mir. 2023. Identification of genetic polymorphism of the MBL2 gene and its association with clinical mastitis in Murrah buffaloes. J. Genet., 102(1): 21. DOI: 10.1007/s1241-023-1419-9
Shi, Y.Y. and L. He. 2005. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res., 15(2): 97-98. DOI: 10.1038/sj.cr.7290272
Wang, C.F., M. Liu, Q.L. Li, Z.H. Ju, J.M. Huang, J.B. Li and J.F. Zhong. 2011. Three novel single-nucleotide polymorphisms of MBL1 gene in Chinese native cattle and their associations with milk performance traits. Vet. Immunol. Immunop., 139(2-4): 229-236. DOI: 10.1016/j.vetimm.2010.10.023
Yeh, F.C., R.C. Yang and T. Boyle. 1999. POPGENE Version 1.32: Microsoft Windows Based Freeware for Population Genetic Analysis, Unviersity of Alberta, Edmonton, Canada.








.png)








