Impact of dextran sulphate adjuentated S. aureus vaccine against the control of mastitis in lactating dairy buffaloes in Pakistan
DOI:
https://doi.org/10.56825/bufbu.2023.4244269Keywords:
Bubalus bubalis, buffaloes, lactating animals, somatic cell count, protein, vaccineAbstract
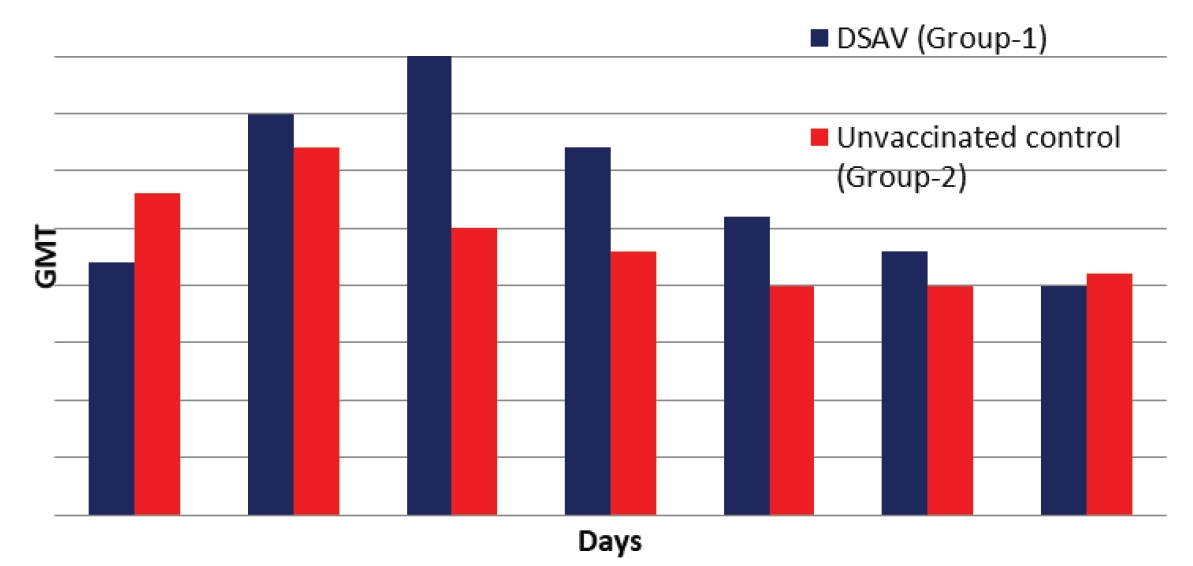
The present study was conducted to evaluate two S. aureus vaccines in 100 mastitis free lactating buffaloes, dividing into 2 equal groups (B1, B2). The animals of B1 and B2 were administered with 2 shots of live attenuated and Dextran sulphate adjuvanted S. aureus vaccine at 15 days sequentially. The evaluation was done with different parameters i.e., serum and whey antibody titers, somatic cell count, milk fat %, milk protein, milk yield, vaccine efficacy, cost-benefit analysis, and colony count. There was a peak of geometric mean antibody titer 291 and 58 in LSAV while its climax 363 and 90 in DSAV at 2 and 6 months of study. In whey this level almost remained the same in both groups. In B1 and B2, somatic cell count kept on decreasing from day zero to the end of study. There was a non-significant difference in milk yield and fat percentage between the 2 groups. Milk protein concentration was significantly different between these groups and was better in B1 than B2. The surf field mastitis test-based quarter point prevalence decreased at 180 days in LASV and DSAV. In California mastitis test based, a significant decreased value was shown in both groups. Pre-vaccination and post-vaccination colony count of S. aureus was more in LSAV than in DSAV. Moreover, the preventative efficacy and cost benefit ratio of DSAV was more excellent as compared to LSAV.
Downloads
Metrics
References
Abdi, R.D., B.E. Gillespie, J. Vaughn, C. Merrill, S.I. Headrick, D.B. Ensermu, D.H. D’Souza, G.E. Agga, R.A. Almeida and S.P. Oliver. 2018. Antimicrobial resistance of staphylococcus aureus isolates from dairy cows and genetic diversity of resistant isolates. Foodborne Pathog. Dis., 15(7): 449-458. DOI: 10.1089/fpd.2017.2362
Akhtar, H., M. Ameer and M. Arshad. 2012. Prevalence of sub-clinical mastitis in Buffaloes at Livestock Research and Development Station, Paharpur and Surroundings of Tehsil Paharpur District D.I. Khan. Pakistan Journal of Science, 64(2): 159-166. DOI: 10.57041/pjs.v64i2.476
Alekish, M.O., K.M. Al-Qudah and A. Al-Saleh. 2013. Prevalence of antimicrobial resistance among bacterial pathogens isolated from bovine mastitis in northern Jordan. Rev. Med. Vet. Toulouse, 164(6): 319-326.
Amer, S., F.L.A. Gálvez and Y. Fukuda. 2018. Prevalence and etiology of mastitis in dairy cattle in El Oro Province, Ecuador. J. Vet. Med. Sci., 80(6): 861-868. DOI: 10.1292/jvms.17-0504
Ashfaq, M., A. Razzaq, S. Haq and M. Ghulam. 2015. Economic analysis of dairy animal diseases in Punjab: A case study of Faisalabad district. J. Anim. Plant Sci., 25(5): 1482-1495.
Athar, M. 2007. Preparation and evaluation of inactivated polyvalent vaccine for the control of mastitis in dairy buffaloes. Ph.D. Dissertation, Department of Veterinary Clinical Medicine and Surgery, University of Agriculture Faisalaba, Faisalabad, Pakistan.
Barkema, H.W., M.J. Green, A.J. Bradley and R.N. Zadoks. 2009. Invited review: The role of contagious disease in udder health. J. Dairy Sci., 92(10): 4717-4729. DOI: 10.3168/jds.2009-2347
Biswadeep, J., P.N. Kumar, S. Abhishek and A. Abrar. 2015. Subclinical bovine mastitis in rural, Peri-Urban and suburban regions of Jaipur district of Rajasthan. Indian J. Anim. Res., 5(1): 175-182. DOI: 10.5958/2277-940X.2015.00028.5
Bradley, A.J., J. Breen, B. Payne, V. White and M.J. Green. 2015. An investigation of the efficacy of a polyvalent mastitis vaccine using different vaccination regimens under field conditions in the United Kingdom. J. Dairy Sci., 98(3): 1706-1720. DOI: 10.3168/jds.2014-8332
Butt, A.A. 2006. Evaluation of four adjuvanted trivalent vaccines for the control of mastitis in dairy buffaloes and cows. Ph.D. Thesis, Faculty of Veterinary Science, University of Agriculture Faisalabad, Faisalabad, Pakistan.
Chishty, M.A., M. Arshad, M. Avais and M. Ijaz. 2007. Cross-sectional epidemiological studies on mastitis in cattle and buffaloes of Tehsil Gojra Pakistan. Bufalo Bull., 26(2): 50-55. Available on: https://kukrdb.lib.ku.ac.th/journal/BuffaloBulletin/search_detail/result/286148
Enger, B.D., C.E. Crutchfield, T.T. Yohe, K.M. Enger, S.C. Nickerson, C.L.M. Parsons and R.M. Akers. 2018. Staphylococcus aureus intramammary challenge in non-lactating mammary glands stimulated to rapidly grow and develop with estradiol and progesterone. Vet. Res., 49(1): 47. DOI: 10.1186/s13567-018-0542-x
El-jakee, J.K., N.E. Aref and A. Gomaa. 2019. Emerging of coagulase negative staphylococci as a cause of mastitis in dairy animals: An environmental hazard. International Journal of Veterinary Science and Medicine, 2(1): 74-78. DOI: 10.1016/j.ijvsm.2013.05.006
Freick, M., Y. Frank, K. Steinert, A. Hamedy, O. Passarge and A. Sobiraj. 2016. Mastitis vaccination using a commercial polyvalent vaccine or a herd-specific Staphylococcus aureus vaccine. Tierärztliche Praxis Großtiere/Nutztiere, 44(4): 219-229. DOI: 10.15653/TPG-150912
Farooq, A.A., S. Inayat, M.S. Akhtar and M. Mushtaq. 2008. Prevalence of mastitis and antibiotic sensitivity of bacterial isolates recovered from Nili Ravi buffaloes. J. Anim. Plant Sci., 18(2-3): 76-77. Available on: https://www.thejaps.org.pk/docs/18_2-3_2008/08-819.pdf
Hussain, A., M.D. Ahmed, M.H. Mushtaq, M.S. Khan, M. Nisar, N. Sabir and S.A. Khan. 2013. Antibiogram analysis of Staphylococcus aureus isolated from mastitic milk samples of buffaloes in district Bhimber Azad Kashmir. Buffalo Bull., 32(SI.2): 1021-1028. Available on: https://kukrdb.lib.ku.ac.th/journal/BuffaloBulletin/search_detail/result/286703
Hernández-Castellano, L.E., S. Wall, R. Stephan, S. Corti and R. Bruckmaier. 2017. Milk somatic cell count, lactate dehydrogenase activity, and immunoglobulin G concentration associated with mastitis caused by different pathogens: A field study. Schweiz Arch.Tierheilkd, 159(5): 283-290. DOI: 10.17236/sat00115
Hoque, M.N., Z.C. Das, A.K. Talukder, M.S. Alam and M.A. Rahman. 2015. Different screening tests and milk somatic cell count for the prevalence of subclinical bovine mastitis in Bangladesh. Trop. Anim. Health Pro., 47(1): 79-86. DOI: 10.1007/s11250-014-0688-0
Ismail, Z.B. 2017. Mastitis vaccines in dairy cows: Recent developments and recommendations of application. Vet. World, 10(9): 1057-1062. DOI: 10.14202/vetworld.2017.1057-1062
Idriss, S., V. Foltys, V. Tancin, K. Kirchnerova, D. Tancinova and K. Zaujec. 2014. Mastitis pathogens and their resistance against antimicrobial agents in dairy cows in Nitra, Slovak. J. Anim. Sci., 47(1): 33-38. Available on: http://www.cvzv.sk/slju/14_1/6_idriss.pdf
Kerro Dego, O., P.A. Pacha, B.E. Gillespie and G.M. Pighetti. 2020. Experimental Staphylococcus aureus mastitis infection model by teat dipping in bacterial culture suspension in dairy cows. Animals, 10(5): 751. DOI: 10.3390/ani10050751
Kusebauch, U., L.E. Hernández-Castellano, S.L. Bislev, R.L. Moritz, C.M. Røntved and E. Bendixen. 2018. Selected reaction monitoring mass spectrometry of mastitis milk reveals pathogen-specific regulation of bovine host response proteins. J. Dairy Sci., 101(7): 6532-6541. DOI: 10.3168/jds.2017-14312
Landin, H., M.J. Mörk, M. Larsson and K.P. Waller. 2015. Vaccination against Staphylococcus aureus mastitis in two Swedish dairy herds. Acta Vet. Scand., 57(1): 81. DOI: 10.1186/s13028-015-0171-6
Leitner, G., N. Yadlin, E. Lubashevsky, E. Ezra, A. Glickman, M. Chaffer, M. Winkler, A. Saran and Z. Trainin. 2003. Development of a Staphylococcus aureus vaccine against mastitis in dairy cows. II. Field trial. Vet. Immunol. Immunop., 93:153-158.
Mbindyo, C.M., G.C. Gitao and C.M. Mulei. 2020. Prevalence, etiology, and risk factors of mastitis in dairy cattle in Embu and Kajiado counties, Kenya. DOI: 10.1155/2020/8831172
Niedziela, D.A., M.P. Murphy, J. Grant, O.M. Keane and F.C. Leonard. 2020. Clinical presentation and immune characteristics in first-lactation Holstein-Friesian cows following intramammary infection with genotypically distinct Staphylococcus aureus strains. J. Dairy Sci., 103(9): 8453-8466. DOI: 10.3168/jds.2019-17433
Mustafa, Y.S., F.N. Awan and T. Zaman. 2014. Prevalence and antibacterial susceptibility in mastitis in buffalo and cow in district Lahore Pakistan. Buffalo Bull., 32(4): 307-314. Available on: https://kukrdb.lib.ku.ac.th/journal/BuffaloBulletin/search_detail/result/286441
Nyman, A.K., C. Fasth and K.P. Waller. 2018. Intramammary infections with different non-aureus staphylococci in dairy cows. J. Dairy Sci., 101(2): 1403-1418. DOI: 10.3168/jds.2017-13467
Philpot, W.N. 2003. A backward glance-A forward look. National Mastitis Council, Inc. 42nd Annual Meeting Proceedings: Fort Worth, Texas, USA. p. 144-155.
Pyorala, S. 2003. Indicators of inflammation in the diagnosis of mastitis. Vet. Res., 34(5): 565-578. DOI: 10.1051/vetres:2003026
Radostits, O.M., C.C. Gay, C.C. Blood and K.W. Hinchcliff. 2007. Veterinary Medicine A Textbook of The Diseases of Cattle, Sheep, Pigs, Goats, and Horses, 10th ed. W.B. Saunders Co., Philadelphia, USA.
Rehman, S.U., M. Athar, A. Shakoor, G. Muhammad and A.A. Butt. 2004. Standardization of indirect haemagglutination test for titration of antibody against Staphylococcus aureus, Streptococcus agalaetiae and Escherichia coli isolated from bubaline mastitis. Int. J. Agric. Biol., 7(3): 441-444. Available on: https://www.fspublishers.org/published_papers/78259_..pdf
Rohmeier, L., W. Petzl, M. Koy, T. Eickhoff, A. Hülsebusch, S. Jander, L. Macias, A. Heimes, S. Engelmann, M. Hoedemaker, H.M. Seyfert, C. Kühn, H.J. Schuberth, H. Zerbe, H.M. Meyerholz. 2020. In vivo model to study the impact of genetic variation on clinical outcome of mastitis in uniparous dairy cows. BMC Vet. Res., 16(1): 1. DOI: 10.1186/s12917-020-2251-8
Sarwar, M., M.A. Khan, M. un-Nisa and Z. Iqbal. 2002. Dairy industry in Pakistan: A scenario. Intl. J. Agric. Biol., 4(3): 420-428. Available on: https://www.fspublishers.org/published_papers/16747_..pdf
Shakoor, A. 2006. Preparation and evaluation of Staphylococcus aureus vaccines for the control of mastitis in dairy buffaloes (Bubalus bubalis). Ph.D. Dissertation, Department of Clinical Medicine and Surgery, University of Agriculture Faisalabad, Faisalabad, Pakistan.
Sharif, A., M. Umer and G. Muhammad. 2009. Mastitis control in dairy production. Pak. Vet. J., 29(3): 145-148.
Suleiman, T.S., E.D. Karimuribo and R.H. Mdegela. 2018. Prevalence of bovine subclinical mastitis and antibiotic susceptibility patterns of major mastitis pathogens isolated in Unguja island of Zanzibar, Tanzania. Trop. Anim. Health Pro., 50(2): 259-266. DOI: 10.1007/s11250-017-1424-3
Tashakkori, N., B. Khoramian and M.F. Moghadam. 2020. Evaluating the effectiveness of two bovine mastitis vaccines and their influences on oxidant and antioxidant capacities of milk. Trop. Anim. Health Pro., 52(3): 1493-1501. DOI: 10.1007/s11250-019-02156-x
Welderufael, B.G., D.J. Koning, L.L.J. Janss, W.F. Franzén and Fikse. 2016. Simultaneous genetic evaluation of simulated mastitis susceptibility and recovery ability using a bivariate threshold sire model. Acta Agr. Scand. A. An., 66(3): 125-134. DOI: 10.1080/09064702.2016.1275761
Wang, D., Z. Wangb, Z. Yan, J. Wub, T. Ali, J. Li, Y. Lv and B. Han. 2015. Bovine mastitis Staphylococcus aureus: Antibiotic susceptibility profile, resistance genes and molecular typing of methicillin-resistant and methicillin-sensitive strains in China. Infect. Genet. Evol., 31: 9-16. DOI: 10.1016/j.meegid.2014.12.039
Yousaf, A., G. Muhammad, S. ur Rahman, M. Siddique and M.Z. Masood. 2009. Effect of montanide adjuvanted Staphylococcus Aureus bacterin-Toxiod on prevalence and incidence of mastitis in cows. Pak. J. Agric. Sci., 46(2): 119-123.








.png)








