PHENOTYPIC AND GENOTYPIC DETECTION OF COLISTIN RESISTANT ESCHERICHIA COLI FROM RAW BUFFALO MEAT IN PAKISTAN
DOI:
https://doi.org/10.56825/bufbu.2025.4425750Keywords:
Bubalus bubalis, buffaloes, E. coli, buffalo meat, colistin, mcr-1 geneAbstract
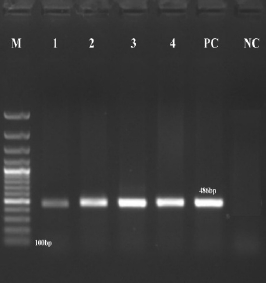
Buffalo meat has been considered as a rich and essential source of proteins for human health. Colistin is considered as the last treatment choice for gram negative bacteria. In this era of modern medicine, the incidence of colistin-resistant Escherichia coli in food, especially meat, has emerged as a significant threat to human health. The present study is planned to detect multidrug resistant E. coli from raw buffalo meat with an emphasis on phenotypic and genotypic detection of colistin resistant E. coli. For this purpose, fresh and chilled raw buffalo meat samples (n=300) were collected and processed for characterization of E. coli. Kirby-Bauer method was used to detect MDR status of E. coli isolates. The colistin broth disc elution test was employed to detect colistin resistance phenotypically while polymerase chain reaction was used to find out genotypic colistin resistance targeting the mcr-1 and mcr-2 genes. The results of the survey conducted revealed that among the total samples collected, 36% (108/300) buffalo meat samples were found positive for E. coli. The highest antibiotic resistance was observed (100%) against tetracycline followed by cefotaxime, cefepime (97.2%) and ciprofloxacin (94.4%). In total, 99 E. coli isolates were detected multidrug resistant and among them 60 (60.60%) isolates were found colistin resistant. The mcr-1 gene was found in 44 (73.33%) colistin resistant isolates while none of the isolates was detected positive for mcr-2 gene. The existence of phenotypic colistin resistance and mcr-1 gene in E. coli isolates from raw buffalo meat is worrisome as this situation leaves us with no treatment options for gram negative pathogens leading the practitioners towards post-antibiotic era.
Downloads
Metrics
References
Abdulhaq, N., Z. Nawaz, M.A. Zahoor and A.B. Siddique. 2020. Association of biofilm formation with multi drug resistance in clinical isolates of Pseudomonas aeruginosa. Excli J., 19: 201-208. DOI: 10.17179/excli2019-2049. DOI: 10.17179/excli2019-2049
Adzitey, F. 2015. Antibiotic resistance of Escherichia coli isolated from beef and its related samples in Techiman municipality of Ghana. Asian J. Anim. Sci., 9(5): 233-240. DOI: 10.1155/2020/8877196
Adzitey, F. 2020. Incidence and antimicrobial susceptibility of Escherichia coli isolated from beef (meat muscle, liver and kidney) samples in Wa Abattoir, Ghana. Cogent Food and Agriculture, 6(1): 718269. DOI: 10.1080/23311932.2020.1718269
Bista, S., T.U. Shrestha, B. Dhungel, P. Koirala, T.R. Gompo, N. Shrestha, N. Adhikari, D.R. Joshi, M.R. Banjara, B. Adhikari, K.R. Rijal and P. Ghimire. 2020. Detection of plasmid-mediated Colistin resistant mcr-1 gene in Escherichia coli isolated from infected chicken livers in Nepal. Animals, 10(11): 2060. DOI: 10.3390/ani10112060
Das, T., M.Z. Islam, E.A. Rana, A. Dutta, S. Ahmed, H. Barua and P.K. Biswas. 2021. Abundance of mobilized colistin resistance gene (mcr-1) in commensal Escherichia coli from diverse sources. Microb. Drug Resist., 27(11): 1585-1593. DOI: 10.1089/mdr.2020.0433
Datta, S., C. Wattal, N. Goel, J.K. Oberoi, R. Raveendran and K.J. Prasad. 2012. A ten year analysis of multi-drug resistant blood stream infections caused by Escherichia coli & Klebsiella pneumoniae in a tertiary care hospital. Indian J. Med. Res., 135(6): 907-912.
Dutta, A., M.Z. Islam, H. Barua, E.A. Rana, M.S. Jalal, P.K. Dhar, A. Das, T. Das, S.M. Sarma, S.K. Biswas and P.K. Biswas. 2020. Acquisition of plasmid mediated colistin resistance gene mcr-1 in Escherichia coli of livestock origin in Bangladesh. Microb. Drug Resist., 26(9): 1058-1062. DOI: 10.1089/mdr.2019.0304
Ewers, C., A. Bethe, T. Semmler, S. Guenther and L.H. Wieler. 2012. Extended-spectrum β-lactamase-producing and AmpC-producing Escherichia coli from livestock and companion animals, and their putative impact on public health: A global perspective. Clin. Microbiol. Infect., 18(7): 646-655. DOI: 10.1111/j.1469-0691.2012.03850.x
Fair, R.J. and Y. Tor. 2014. Antibiotics and bacterial resistance in the 21st century. Perspectives in Medicinal Chemistry, 6: 25-64. DOI: 10.4137/PMC.S14459
Gallardo, P., M. Izquierdo, R.M. Vidal, N. Chamorro-Veloso, R. Rosselló-Móra, M. O’Ryan and M.J. Farfán. 2017. Distinctive gut microbiota is associated with diarrheagenic Escherichia coli infections in children. Front. Cell Infect. Mi., 7: 424. DOI: 10.3389/fcimb.2017.00424
Ilyas, S., M.U. Qamar, M.H. Rasool, N. Abdulhaq and Z. Nawaz. 2016. Multidrug-resistant pathogens isolated from ready-to-eat salads available at a local market in Pakistan. Brit. Food J., 118(8): 2068-2075. DOI: 10.1108/BFJ-02-2016-0058
Islam, A., Z. Rahman, S. Monira, A. Rahman, A. Camilli, C.M. George, N. Ahmed and M. Alam. 2017. Colistin resistant Escherichia coli carrying mcr- 1 in urban sludge samples: Dhaka, Bangladesh. Gut Pathog., 9(1): 77. DOI: 10.1186/s13099-017-0227-4
Javed, H., S. Saleem, A. Zafar, A. Ghafoor, A.B. Shahzad, H. Ejaz, K. Junaid and S. Jahan. 2020. Emergence of plasmid‑mediated mcr genes from Gram‑negative bacteria at the human‑animal interface. Gut Pathog., 12(1): 54. DOI: 10.1186/s13099-020-00392-3
Ling, Z., W. Yin, Z. Shen, Y. Wang, J. Shen and T.R. Walsh. 2020. Epidemiology of mobile colistin resistance genes mcr-1 to mcr-9. J. Antimicrob. Chemoth., 75: 3087-3095. DOI: 10.1093/jac/dkaa205
Liu, Y. and J.H. Liu. 2018. Monitoring colistin resistance in food animals, an urgent threat. Expert Rev. Anti.-Infe., 16(6): 443-446. DOI: 10.1016/S1473-3099(15)00424-7
Liu, Y.Y., Y. Wang, T.R. Walsh, L.X. Yi, R. Zhang, J. Spencer, Y. Doi, G. Tian, B. Dong, X. Huang, L. Yu, D. Gu, H. Ren, X. Chen, L. Lv, D. He, H. Zhou, Z. Liang, J. Liu and J. Shen. 2016. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis., 16(2): 161-168. DOI: 10.1016/S1473-3099(15)00424-7
Mgaya, F.X., M.I. Matee, A.P. Muhairwa and A.S. Hoza. 2021. Occurrence of multidrug resistant Escherichia coli in raw meat and cloaca swabs in poultry processed in slaughter slabs in Dar es Salaam, Tanzania. Antibiotics, 10(4): 343. DOI: 10.3390/antibiotics10040343
Mohsin, M., S. Raza, N. Roschanski, S. Guenther, A. Ali and P. Schierack. 2017. Description of the first Escherichia coli clinical isolate harboring the colistin resistance gene mcr-1 from the Indian subcontinent. Antimicrob. Agents Ch., 61(1): e01945. DOI: 10.1128/AAC.01945-16
Muktan, B., T.U. Shrestha, B. Dhungel, B.C. Mishra, N. Shrestha, N. Adhikari, M.R. Banjara, B. Adhikari, K.R. Rijal and P. Ghimire. 2020. Plasmid mediated colistin resistant mcr‑1 and co‑existence of OXA‑48 among Escherichia coli from clinical and poultry isolates: first report from Nepal. Gut Pathog., 12: 44. DOI: 10.1186/s13099-020-00382-5
Nagappa, K., S. Tamuly, Brajmadhuri, M.K. Saxena and S.P. Singh. 2007. Isolation of Salmonella typhimurium from poultry eggs and meat of Tarai region of Uttaranchal. Indian J. Biotechnol., 6(3): 407-409.
Nawaz, Z., B. Aslam, M.A. Zahoor, A.B. Siddique, A. Rafique, R. Aslam, M.U. Qamar, S. Ali and M.U. Mubeen. 2021. Frequency of Extended Spectrum Beta Lactamase producing Escherichia coli in fresh and frozen meat. Pak. Vet. J., 41(1): 102-106. DOI: 10.29261/pakvetj/2020.059
Nawaz, Z., M.K. Zahoor, A.B. Siddique, B. Aslam, S. Muzammil, A. Yasmin, I. Fayyaz and M.K. Zahoor. 2019. Molecular identification of blaCTX-M and blaTEM genes among multi-drug resistant Enteropathogenic Escherichia coli isolated from children. Pak. J. Pharm. Sci., 32(Suppl. 3): 1215-1218.
Nishino, Y., Y. Shimojima, Y. Suzuki, M. Ida, R. Fukui, S. Kuroda, A. Hirai and K. Sadamasu. 2017. Detection of the mcr-1 gene in colistin-resistant Escherichia coli from retail meat in Japan. Microbiol. Immunol., 61(12): 554-557. DOI: 10.1111/1348-0421.12549
Poirel, L. and P. Nordmann. 2016. Emerging plasmid-encoded colistin resistance: the animal world as the culprit? J. Antimicrob. Chemoth., 71(8): 2326-2327. DOI: 10.1093/jac/dkw074
Rahman, S.U., S. Ahmad and I. Khan. 2019. Incidence of ESBL-producing-Escherichia coli in poultry farm environment and retail poultry meat. Pak. Vet. J., 39(1): 116-120. DOI: 10.29261/pakvetj/2018.091
Ranjan, A., S. Shaik, N. Nishant, A. Hussain, S.K. Tiwari, T. Semmler, S. Jadhav, L. H. Wieler, M. Alam, R.R. Colwell and N. Ahmed. 2017. Comparative genomics of Escherichia coli isolated from skin and soft tissue and other extraintestinal Infections. MBio, 8(4): e01070-17. DOI: 10.1128/mBio.01070-17
Rasheed, M.U., N. Thajuddin, P. Ahamed, Z. Teklemariam and K. Jamil. 2014. Antimicrobial drug resistance in strains of Escherichia coli isolated from food sources. Rev. I. Med. Trop., 56(4): 341-346. DOI: 10.1590/s0036-46652014000400012
Rebelo, A.R., V. Bortolaia, J.S. Kjeldgaard, S.K. Pedersen, P. Leekitcharoenphon, I.M. Hansen, B. Guerra, B. Malorny, M. Borowiak, J.A. Hammerl, A. Battisti, A. Franco, P. Alba, A.P. Guyomad, S.A. Granier, C.F. Escobar, S.M. Kumar, L. Villa, A. Caratolli and R.S. Hendriksen. 2018. Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Eurosurveillance, 23(6): 17-00672. DOI: 10.2807/1560-7917.ES.2018.23.6.17-00672
Saud, B., G. Paudel, S. Khichaju, D. Bajracharya, G. Dhungana, M.S. Awasthi and V. Shrestha. 2019. Multidrug-Resistant bacteria from raw meat of buffalo and chicken, Nepal. Veterinary Medicine International, DOI: 10.1155/2019/7960268
Sethulekshmi, C., C. Latha and B. Sunil. 2016. Occurrence of Enterohaemorrhagic E. coli in raw meat samples in Kerala. International Journal of Advanced Research in Biological Sciences, 3: 220-222.
Shafiq, M., J. Huang, S. Ur Rahman, J.M. Shah, L. Chen, Y. Gao, M. Wang and L. Wang. 2019. High incidence of multidrug-resistant Escherichia coli coharboring mcr-1 and bla CTX-M-15 recovered from pigs. Infection and Drug Resistance, 12: 2135-2149. DOI: 10.2147/IDR.S209473
Shafiq, M., S.U. Rahman, H. Bilal, A. Ullah, S.M. Noman, M. Zeng, Y. Yuan, Q. Xie, X. Li and X. Jiao. 2022. Incidence and molecular characterization of ESBL-producing and colistin-resistant Escherichia coli isolates recovered from healthy food-producing animals in Pakistan. J. Appl. Microbiol., 133(3): 1169-1182. DOI: 10.1111/jam.15469
Simner, P.J., Y. Bergman, M. Trejo, A.A. Roberts, R. Marayan, T. Tekle, S. Campeau, A.Q. Kazmi, D.T. Bell, S. Lewis, P.D. Tamma, R. Humppries and J.A. Hindler. 2019. Two-site evaluation of the colistin broth disk elution test to determine colistin in vitro activity against gram-negative bacilli. J. Clin. Microbiol., 57(2): e01163. DOI: 10.1128/JCM.01163-18Skov, R.L. and D.L. Monnet. 2016. Plasmid-mediated colistin resistance (mcr-1 gene): Three months later, the story unfolds. Eurosurveillance, 21(9): 30155. DOI: 10.2807/1560-7917.ES.2016.21.9.30155.
Uddin, M.B., M.N. Alam, M. Hasan, S.M.B. Hossain, M. Debnath, R. Begum, M.A. Samad, S.F. Hoque, M.S.R. Chowdhury, M.M. Rahman, M. Hossain, M.M. Hassan, A. Lundkvist, J.D. Jarhult, M.E. Zowalatay and S.S.U. Ahmed. 2022. Molecular detection of Colistin resistance mcr-1 gene in multidrug-resistant Escherichia coli isolated from chicken. Antibiotics, 11(1): 97. DOI: 10.3390/antibiotics11010097
Wang, R., L. van Dorp, L.P. Shaw, P. Bradley, Q. Wang, X. Wang, L. Jin,Q. Zhang, Y. Liu, A. Rieux, T.D. Schneiders, L.A. Weinert, Z. Iqbal, X. Didelot, H. Wang and F. Balloux. 2018. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun., 9(1): 1179. DOI: 10.1016/j.ijantimicag.2018.01.023
Wickramasinghe, N.N., J. Ravensdale, R. Coorey, S.P. Chandry and G.A. Dykes. 2017. The predominance of psychrotrophic Pseudomonads on aerobically stored chilled red meat. Compr. Rev. Food Sci. F., 18(5): 1622-1635. DOI: 10.1111/1541-4337.12483
Zajac, M., P. Sztromwasser, V. Bortolaia, P. Leekitcharoenphon, P., L.M. Cavaco, A. Ziȩtek-Barszcz, R.S. Hendriksen and D. Wasyl. 2019. Occurrence and characterization of mcr-1-positive Escherichia coli isolated from food-producing animals in Poland, 2011-2016. Front. Microbiol., 10: 1753. DOI: 10.3389/fmicb.2019.01753
Zulqarnain, M., N. Sarwar, A.A. Anjum, S. Firyal, T. Yaqub and M. Rabbani. 2021. Molecular detection of colistin resistance gene (MCR-1) in E. coli isolated from cloacal swabs of broilers. Pak. Vet. J., 41(2): 284-288. DOI: 10.29261/pakvetj/2021.016








.png)








