ผลของสารสกัดโปรตีนไหม (sericin) ต่อคุณภาพของน้ำเชื้อช้างเอเชีย (Elephas maximus) แช่แข็ง ภายหลังการอุ่นละลาย Effect of Sericin Supplementation on Post-Thawed Asian Elephant (Elephas maximus) Semen Quality
##plugins.themes.bootstrap3.article.main##
บทคัดย่อ
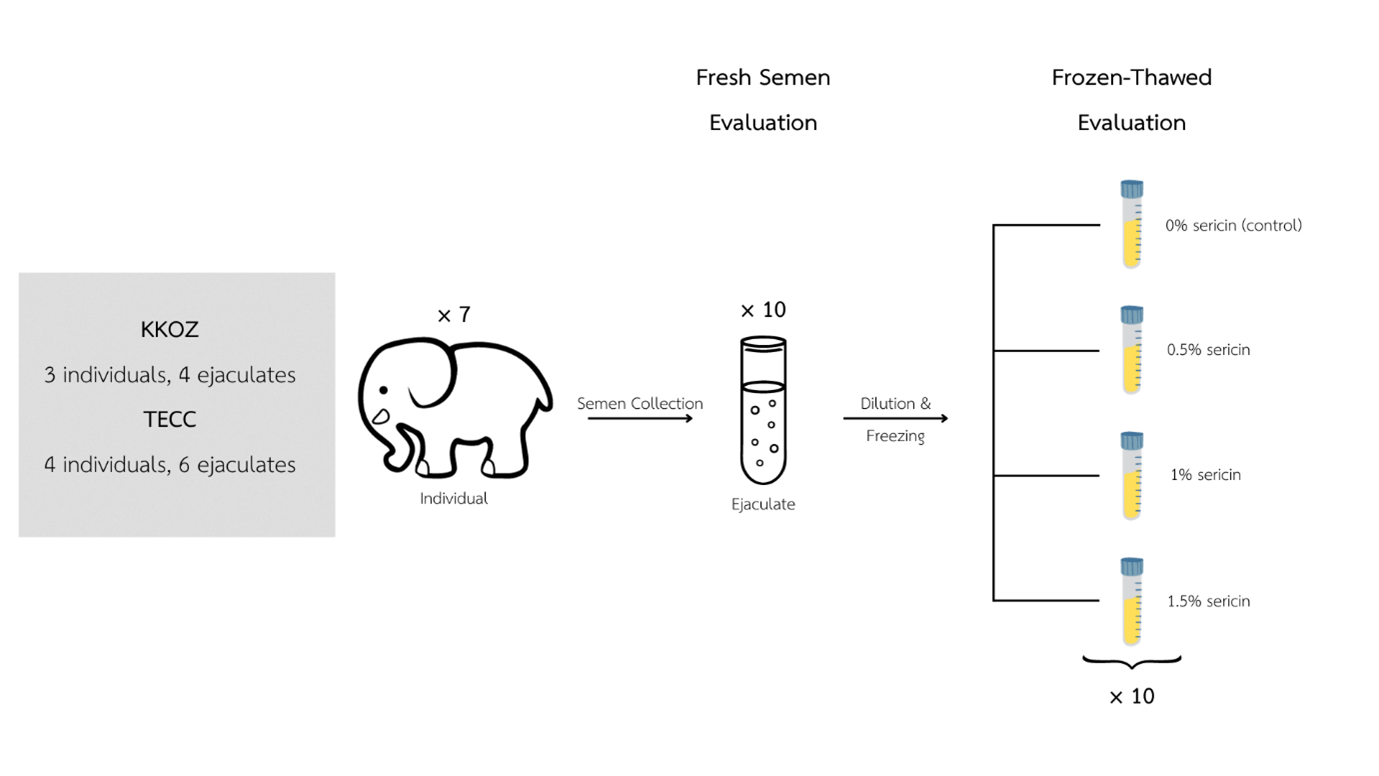
The development of optimal components for cryopreservation medium is critical for breeding management and genetic diversity preservation in endangered species like the Asian elephant (Elephas maximus). Sericin, a silk protein, has emerged as a novel cryoprotectant due to its antioxidative, membrane-stabilizing, and anti-apoptotic properties. This study evaluated the influence of sericin supplementation as a cryoprotectant on post-thaw Asian elephant sperm quality. Ten ejaculates were collected, diluted in a TEST with 20% egg yolk and 5% glycerol extender accompanied with varying sericin concentrations (0%, 0.5%, 1%, 1.5%), cryopreserved, and evaluated to determine post-thawed motility, viability, structural stability of the membrane and DNA, and motility parameters. The statistical analysis indicated notable enhancements in straightness and viability with 1.5% and 1% sericin, respectively, in comparison to the control group with p-value < 0.05. Based on previous findings in sericin studies involving different species, this investigation confirms that the inclusion of 1-1.5% sericin in freezing solutions improves specific parameters such as velocity, straightness, and viability of post-thaw Asian elephant sperm. Further research should clarify the exact mechanisms between semen-specific improvements and fertilizing ability according to the sericin supplementation in semen extender.
บทคัดย่อ
การพัฒนาองค์ประกอบสารละลายเพื่อการรักษาสภาพในระหว่างการแช่แข็งยังคงมีความจำเป็นต่อการจัดการความหลากหลายทางพันธุกรรมในสัตว์ที่อยู่ในภาวะใกล้สูญพันธุ์ อาทิเช่น ช้างเอเชีย (Elephas maximus) โดยจากการศึกษาในสปีชีส์อื่นก่อนหน้านี้ เซริซิน (sericin) ให้ผลการทดลองในการคงคุณภาพน้ำเชื้อภายหลังผ่านกระบวนการแช่แข็งผ่านกลไกการต่อต้านอนุมูลอิสระ (antioxidative), คงสภาพเยื่อหุ้มเซลล์ของสเปิร์ม (membrane-stabilizing), และลดการถูกทำลายของเซลล์ (anti-apoptotic) ดังนั้น การศึกษาครั้งนี้จึงจัดทำขึ้นเพื่อวิเคราะห์ประสิทธิภาพของเซริซินที่ความเข้มข้นต่างๆ (0%, 0.5%, 1%, 1.5%) ในการคงสภาพตัวอย่างน้ำเชื้อช้างเอเชีย 10 ตัวอย่าง จากช้าง 7 เชือก ระหว่างการเก็บรักษาด้วยการแช่แข็งร่วมกับสารละลายมาตรฐานสูตร TEST 20% egg-yolk และ 5% glycerol โดยตรวจประเมินคุณภาพการเคลื่อนที่, สเปิร์มที่มีชีวิต (viability), ความสมบูรณ์ของอะโครโซม (acrosome), ความสมบูรณ์ของ DNA, และความสมบูรณ์ของเยื่อหุุ้มเซลล์ (plasma membrane) หลังผ่านการวิเคราะห์ทางสถิติพบความแตกต่างอย่างมีนัยสำคัญที่ความตรงของการเคลื่อนที่ (straightness) และร้อยละของสเปิร์มที่มีชีวิต ที่ความเข้มข้นของเซริซิน 1.5% และ 1% ตามลำดับ เมื่อเปรียบเทียบกับการทดลองในสปีชีส์อื่นๆจึงสนับสนุนได้ว่าเซริซินที่ความเข้มข้น 1% และ 1.5% สามารถช่วยคงคุณภาพน้ำเชื้อในกระบวนการแช่แข็งได้ อย่างไรก็ตามแม้มีการคาดการณ์กลไกการทำงานของเซริซินในทางชีวเคมี แต่ยังคงมีความจำเป็นในการศึกษาเพื่อระบุการทำงานที่ชัดเจนของเซริซินต่อไป
##plugins.generic.usageStats.downloads##
##plugins.themes.bootstrap3.article.details##
เอกสารอ้างอิง
Aghaz, F., Khazaei, M., Vaisi-Raygani, A., & Bakhtiyari, M. (2020). Cryoprotective effect of sericin supplementation in freezing and thawing media on the outcome of cryopreservation in human sperm. The Aging Male, 23(5), 469-476. doi:10.1080/13685538.2018.1529156.
Bailey, J. L., Bilodeau, J. F., & Cormier, N. (2000). Semen cryopreservation in domestic animals: a damaging and capacitating phenomenon. Journal of Andrology, 21(1), 1-7. doi: 10.1002/j.1939-4640.2000.tb03268.x.
Björndahl, L., Söderlund, I., & Kvist, U. (2003). Evaluation of the one-step Eosin–Nigrosin staining technique for human sperm vitality assessment. Human reproduction (Oxford, England), 18(4), 813-816. doi:10.1093/humrep/deg199.
Cho, K. Y., Moon, J. Y., Lee, Y. W., Lee, K. G., Yeo, J. H., Kweon, H. Y., . . . Cho, C. S. (2003). Preparation of self-assembled silk sericin nanoparticles. International Journal of Biological Macromolecules, 32(1-2), 36-42. doi:10.1016/s0141-8130(03)00023-0.
Funahashi, H., & Sano, T. (2005). Select antioxidants improve the function of extended boar semen stored at 10 degrees C. Theriogenology, 63(6), 1605-1616. doi:10.1016/j.theriogenology.2004.06.016.
Hermes, R., Göritz, F., Streich, W., & Hildebrandt, T. (2007). Assisted Reproduction in Female Rhinoceros and Elephants – Current Status and Future Perspective. Reproduction in Domestic Animals, 42(2), 33-44. doi:10.1111/j.1439-0531.2007.00924.x
Imrat, P., Suthanmapinanth, P., Saikhun, K., Mahasawangkul, S., Sostaric, E., Sombutputorn, P., Stout, T. A. (2013). Effect of pre-freeze semen quality, extender and cryoprotectant on the post-thaw quality of Asian elephant (Elephas maximus indicus) semen. Cryobiology, 66(1), 52-59. doi:10.1016/j.cryobiol.2012.11.003
Isobe, T., Ikebata, Y., Onitsuka, T., Do, L. T., Sato, Y., Taniguchi, M., & Otoi, T. (2013). Cryopreservation for bovine embryos in serum-free freezing medium containing silk protein sericin. Cryobiology, 67(2), 184-187. doi:10.1016/j.cryobiol.2013.06.010
Isobe, T., Ikebata, Y., Onitsuka, T., Wittayarat, M., Sato, Y., Taniguchi, M., & Otoi, T. (2012). Effect of sericin on preimplantation development of bovine embryos cultured individually. Theriogenology, 78(4), 747-752. doi:10.1016/j.theriogenology.2012.03.021
Jeyendran, R. S., Van der Ven, H. H., Perez-Pelaez, M., Crabo, B. G., & Zaneveld, L. J. (1984). Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. Journal of Reproduction and Fertility, 70(1), 219-228. doi:10.1530/jrf.0.0700219
Jeyendran, R. S., Van der Ven, H. H., & Zaneveld, L. J. (1992). The hypoosmotic swelling test: an update. Archives of Andrology, 29(2), 105-116. doi:10.3109/01485019208987714.
Jones, R., Mann, T., & Sherins, R. (1979). Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil Steril, 31(5), 531-537. doi:10.1016/s0015-0282(16)43999-3.
Kadirvel, G., Kumar, S., & Kumaresan, A. (2009). Lipid peroxidation, mitochondrial membrane potential and DNA integrity of spermatozoa in relation to intracellular reactive oxygen species in liquid and frozen-thawed buffalo semen. Animal Reproduction Science, 114(1-3), 125-134. doi:10.1016/j.anireprosci.2008.10.002.
Kato, N., Sato, S., Yamanaka, A., Yamada, H., Fuwa, N., & Nomura, M. (1998). Silk Protein, Sericin, Inhibits Lipid Peroxidation and Tyrosinase Activity. Bioscience, Biotechnology, and Biochemistry, 62(1), 145-147. doi:10.1271/bbb.62.145.
Khiabani, A. B., Moghaddam, G., & Kia, H. D. (2017). Effects of adding different levels of Glutamine to modified Beltsville extender on the survival of frozen rooster semen. Animal Reproduction Science, 184, 172-177. doi: 10.1016/j.anireprosci.2017.07.013.
Kiso, W. K., Asano, A., Travis, A. J., Schmitt, D. L., Brown, J. L., & Pukazhenthi, B. S. (2012). Pretreatment of Asian elephant (Elephas maximus) spermatozoa with cholesterol-loaded cyclodextrins and glycerol addition at 4 degrees C improves cryosurvival. Reproduction, Fertility and Development, 24(8), 1134-1142. doi:10.1071/RD11266.
Kiso, W. K., Brown, J. L., Siewerdt, F., Schmitt, D. L., Olson, D., Crichton, E. G., & Pukazhenthi, B. S. (2011). Liquid semen storage in elephants (Elephas maximus and Loxodonta africana): species differences and storage optimization. Journal of Andrology, 32(4), 420-431. doi:10.2164/jandrol.110.011460
Kiso, W. K., Selvaraj, V., Nagashima, J., Asano, A., Brown, J. L., Schmitt, D. L., Pukazhenthi, B. S. (2013). Lactotransferrin in Asian elephant (Elephas maximus) seminal plasma correlates with semen quality. Plos One, 8(8), e71033. doi:10.1371/journal.pone.
Kumar, P., Kumar, D., Sikka, P., & Singh, P. (2015). Sericin supplementation improves semen freezability of buffalo bulls by minimizing oxidative stress during cryopreservation. Animal Reproduction Science, 152, 26-31. doi:10.1016/j.anireprosci.2014.11.015.
Larson, J. L., & Miller, D. J. (1999). Simple histochemical stain for acrosomes on sperm from several species. Molecular Reproduction and Development, 52(4), 445-449. doi:10.1002/(sici)1098-2795(199904)52:4<445::Aid-mrd14>3.0.Co;2-6.
Macfarlane, J. S. (1991). Salamon's artificial insemination of sheep and goats. Tropical animal health and production, 23(2), 114-114. doi:10.1007/BF02361195.
Marti, E., Mara, L., Marti, J. I., Muiño-Blanco, T., & Cebrián-Pérez, J. A. (2007). Seasonal variations in antioxidant enzyme activity in ram seminal plasma. Theriogenology, 67(9), 1446-1454. doi:10.1016/j.theriogenology.2007.03.002
Maxwell, W. M. C., & Watson, P. F. (1996). Recent progress in the preservation of ram semen. Animal Reproduction Science, 42(1), 55-65. doi: 10.1016/0378-4320(96)01544-8.
Michael, A. J., Alexopoulos, C., Pontiki, E. A., Hadjipavlou-Litina, D. J., Saratsis, P., Ververidis, H. N., & Boscos, C. M. (2009). Effect of antioxidant supplementation in semen extenders on semen quality and reactive oxygen species of chilled canine spermatozoa. Anim Reprod Sci, 112(1-2), 119-135. doi:10.1016/j.anireprosci.2008.04.007.
Miyamoto, Y., Oishi, K., Yukawa, H., Noguchi, H., Sasaki, M., Iwata, H., & Hayashi, S. (2012). Cryopreservation of human adipose tissue-derived stem/progenitor cells using the silk protein sericin. Cell Transplant, 21(2-3), 617-622. doi:10.3727/096368911x605556.
O'Brien, J. K., Steinman, K. J., Montano, G. A., Love, C. C., & Robeck, T. R. (2013). Sperm DNA fragmentation and morphological degeneration in chilled elephant (Elephas maximus and Loxodonta Africana) semen collected by transrectal massage. Andrology, 1(3), 387-400. doi:10.1111/j.2047-2927.2013.00080.x.
Ohnishi, K., Murakami, M., Morikawa, M., & Yamaguchi, A. (2012). Effect of the silk protein sericin on cryopreserved rat islets. Journal of Hepato-Biliary-Pancreatic Sciences, 19(4), 354-360. doi:10.1007/s00534-011-0415-4.
Pinyopummin, A., Mahasawangkul, S., Nunklang, G., Kornkaewrat, K., Laopiem, S., Koonjaenak, S., & Wattananit, P. (2018). Supplemented stallion seminal plasma can improve impaired motility due to the dilution effect in chilled Asian elephant sperm. Reproduction in Domestic Animals, 53(2), 525-533. doi:10.1111/rda.13141.
Ratchamak, R., Authaida, S., Boonkum, W., & Chankitisakul, V. (2023). Improvement of rooster semen freezability and fertility rate after sericin supplementation in freezing semen extender. Animal Bioscience, 36(10), 1530-1535. doi:10.5713/ab.23.0016.
Ratchamak, R., Ratsiri, T., Kheawkanha, T., Vongpralub, T., Boonkum, W., & Chankitisakul, V. (2020). Evaluation of cryopreserved boar semen after supplementation sericin form silkworm (Bombyx mori) in semen extender. Animal Science Journal, 91(1), e13428. doi: 10.1111/asj.13428.
Raza, S., Uçan, U., Aksoy, M., Erdoğan, G., Ceylan, A., & Serin, I. (2019). Silk protein sericin pretreatment enhances osmotic tolerance and post-thaw sperm quality but reduces the ability of sperm cells to undergo in vitro induced acrosome reaction in rabbit. Cryobiology, 90, 1-7. doi: 10.1016/j.cryobiol.2019.09.008.
Sasaki, M., Kato, Y., Yamada, H., & Terada, S. (2005). Development of a novel serum-free freezing medium for mammalian cells using the silk protein sericin. Biotechnology and Applied Biochemistry, 42(2), 183-188. doi:10.1042/BA20050019.
Satitmanwiwat, S., Promthep, K., Buranaamnuay, K., Mahasawangkul, S., & Saikhun, K. (2017). Lipid and protein oxidation levels in spermatozoa and seminal plasma of Asian Elephants (Elephas maximus) and their relationship with semen parameters. Reproduction in Domestic Animals, 52(2), 283-288. doi:10.1111/rda.12900.
Schmitt, D. L., & Hildebrandt, T. B. (1998). Manual collection and characterization of semen from Asian elephants (Elephas maximus). Animal Reproduction Science, 53(1), 309-314. doi: 10.1016/S0378-4320(98)00120-1.
Takahashi, M., Tsujimoto, K., Yamada, H., Takagi, H., & Nakamori, S. (2003). The silk protein, sericin, protects against cell death caused by acute serum deprivation in insect cell culture. Biotechnology Letters,25(21),1805-1809.doi:10.1023/A:1026284620236.
Takechi, T., Wada, R., Fukuda, T., Harada, K., & Takamura, H. (2014). Antioxidant activities of two sericin proteins extracted from cocoon of silkworm (Bombyx mori) measured by DPPH, chemiluminescence, ORAC and ESR methods. Biomedical Reports,2(3),364-369.doi:10.3892/br.2014.244.
Tao, W., Li, M., & Xie, R. (2005). Preparation and Structure of Porous Silk Sericin Materials. Macromolecular Materials and Engineering, 290(3), 188-194. doi: 10.1002/mame.200400306.
Tejada, R. I., Mitchell, J. C., Norman, A., Marik, J. J., & Friedman, S. (1984). A test for the practical evaluation of male fertility by acridine orange (AO) fluorescence. Fertility and Sterility, 42(1), 87-91. doi:10.1016/s0015-0282(16)47963-x.
Thongphakdee, A., Kiatsomboon, S., Noimoon, S., Kongprom, U., Boonorana, I., Karoon, S., Thongtip, N. (2022). Semen characteristics and second successful artificial insemination of Asian elephant (Elephas maximus) in Thailand. Veterinary World, 15(5), 1246-1255. doi: 10.14202/vetworld.2022.1246-1255.
Thongtip, N., Saikhun, J., Damyang, M., Mahasawangkul, S., Suthunmapinata, P., Yindee, M., Pinyopummin, A. (2004). Evaluation of post-thaw Asian elephant (Elephas maximus) spermatozoa using flow cytometry: the effects of extender and cryoprotectant. Theriogenology, 62(3-4),748-760.doi:10.1016/j.theriogenology.2003.11.021.
Thongtip, N., Saikhun, J., Mahasawangkul, S., Kornkaewrat, K., Pongsopavijitr, P., Songsasen, N., & Pinyopummin, A. (2008). Potential factors affecting semen quality in the Asian elephant (Elephas maximus). Reproductive Biology and Endocrinology, 6(9). doi:10.1186/1477-7827-6-9.
Thongtipsiridech, S., Imrat, P., Srihawong, T., Mahasawangkul, S., Tirawattanawanich, C., & Saikhun, K. (2013). Seminal Plasma MDA Concentrations Correlating Negatively with Semen Quality in Asian elephants. The Thai Journal of Veterinary Medicine, 41(2), 199-204.
Wattananit, P., Mahasawangkul, S., Gosalvez, J., Suthanmapinanth, P., Sombutputorn, P., Jansittiwate, S., Stout, T. (2012). Effect of cooled storage on quality and DNA integrity of Asian elephant (Elephas maximus) spermatozoa. Reproduction, Fertility and Development, 24(8), 1105-1116. doi: 10.1071/RD11309.
Williams, C., Tiwari, S.K., Goswami, V.R., de Silva, S., Kumar, A., Baskaran, N., Yoganand, K. & Menon, V. (2020). Elephas maximus. Retrieved February 19, 2022, from https://www.iucnredlist.org/species/7140/45818198
Yangngam, Y., Chapanya, S., Vongpralub, T., Boonkum, W., & Chankitisakul, V. (2021). Effect of semen extender supplementation with sericin on post-thaw dairy bull sperm quality and lipid peroxidation. Czech Journal of Animal Science, 66(1), 13-20.